All published articles of this journal are available on ScienceDirect.
HSV-2 Induced Cerebellitis: A Case Report of HSV-2 Reactivation by SARS-CoV-2 Infection
Abstract
Background:
Herpes simplex virus type 2 rarely causes encephalitis in humans. Some DNA viruses, such as HSV-1 and HSV-2, can be reactivated by COVID-19 infection. SARS-CoV-2 causes a wide spectrum of neurological deficits, such as stroke, delirium, movement disorders, and neuropathy.
Case Presentation:
An unusual manifestation of HSV-2 was diagnosed as cerebellitis in our patient. It was concluded that SARS-CoV-2 can reactivate DNA viruses, such as HSV-2. Here, we reported a 1-year-old female infant with cerebellitis due to herpes simplex virus type 2 infection.
Conclusion:
The patient was treated with intravenous acyclovir and oral prednisolone for three weeks. Finally, during her 9-month neurological follow-up, she was able to walk with minimal ataxia.
1. INTRODUCTION
Herpes simplex virus type 2 rarely causes encephalitis in humans. Some DNA viruses, such as HSV-1 and HSV-2, can be reactivated by COVID-19 infection. SARS-CoV-2 causes a wide spectrum of neurological deficits, such as stroke, delirium, movement disorders, and neuropathy like Guillain-Barré syndrome (GBS).
In an article by Chen et al., the reactivation of human herpesviruses in COVID-19 patients and vaccinated people has been reported in 2022. They mentioned that SARS-CoV-2 coinfection, COVID-19 treatments, and vaccination might be involved in those herpesvirus-associated diseases by reactivation of viruses in latently infected host cells [1].
In this case presentation, an unusual manifestation of HSV-2 was diagnosed as cerebellitis in this patient. We concluded that SARS-CoV-2 could reactivate DNA viruses, such as HSV-2.
2. CASE PRESENTATION
A one-year-old girl was presented by her parents to the emergency department with sudden onset of acute truncal ataxia without any complications in consciousness, visual acuity, upper and lower extremities' forces, and deep tendon reflexes. No signs of increased intracranial pressure were detected simultaneously. An emergency MRI of the brain without contrast showed multiple abnormal signals (4 high signals measuring 2 x 1 cm on T2 and FLAIR sequences) in the cerebellum favouring the cerebellitis (Fig. 1). In her past medical history, she was delivered by caesarean section with no insults during delivery. Parents were consanguineous. She was admitted to the pediatric neurology department for further evaluation and complimentary evaluations.
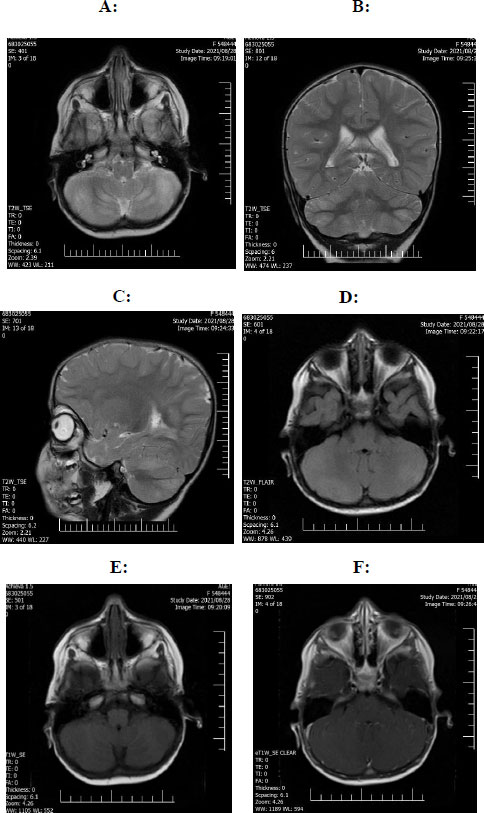
(D): Axial Flair sequence reveals high signal intensity in bilateral cerebellar hemispheric hyperintensity.
(E): Axial T1 sequence reveals bilateral cerebellar hemisphere hypointensity.
(F): Axial T1 sequences with GAD show partial enhancement in bilateral cerebellar hemispheric involvement.
Most recently, she was diagnosed with SARS-CoV-2 virus approximately three weeks before admission. COVID-19 PCR was negative at the time of admission, but the COVID-IgG antibody test was reported positive: 3.32 units/millilitres (Negative: less than 0.9), while the IgM antibody was negative: 0.12 units/ millilitres (Negative: less than 0.9). COVID IgG result of more than 1.1 units/ millilitres was detected as positive according to the laboratory references. A lumbar puncture was done and revealed an increase in white blood cells with lymphocyte dominancy and an increase in protein. The CSF pressure was within normal limits. Antibiotics and acyclovir were started due to the presence of white blood cells in the spinal fluid, and a repeat spinal fluid sample was sent to check common viral infections by PCR method. The result of the spinal fluid examination was negative for bacterial meningitis. In MRI, high signal intensity in bilateral cerebellar hemispheric was seen.
Molecular analysis reports by PCR for EBV, VZV, CMV, HSV 1, and HHV 6,7,8 were undetectable, but PCR for HSV 2 virus was positive. IgM and IgG serum HSV 2 antibodies were also positive. During treatment with acyclovir, she received IVIG (two grams per kilogram) according to recent literature recommendations for HSV cerebellitis, but no improvement in ataxia was seen. As a subsequent treatment with a dramatic response, a high dose of methylprednisolone (20 mg/Kg) was prescribed for 5 days and continued with a maintenance dose. The patient was treated with intravenous acyclovir and oral prednisolone for three weeks in hospital. The second HSV DNA PCR [1, 2] was reported undetectable in CSF following treatment with acyclovir. EEG was performed to detect any epileptiform discharge or background slowing, but no abnormality in favour of encephalitis was detected. Finally, she was discharged with mild ataxia.
During her neurological follow-ups in a 9-month period of time, she was able to walk with minimal ataxia and a wide-based gait.
3. DISCUSSION
All herpesviruses have double-stranded DNA, including human herpes simplex virus type 1 (HSV-1) and type 2 (HSV-2), varicella-zoster virus, Epstein-Barr virus, cytomegalovirus, HHV-6, HHV-7, and HHV-8.
The herpesvirus family has latency and reactivation periods that can be triggered by some factors, like stress, trauma, fever, and illness [2, 3]. However, herpesviruses cause central nervous system infection through primary infection. They can also reactivate and lead to infection from a latent state. Since herpes encephalitis entails mortality and morbidity in children and adults, it is of high importance to diagnose it promptly. HSV-2 is difficult to diagnose, unlike HSV-1 CNS infection based on the temporal lobe involvement. Therefore, it needs to perform lumbar puncture and HSV PCR on CSF fluid. HSV-2 usually has atypical radiographic findings [4, 5]. CNS complications of HSV-2 infection are more common in immunocompromised patients, especially in HIV infection [6-8]. Pelligra et al. studied neonatal HSV-2 encephalitis. They declared that it could be multifocal or limited to only the temporal lobes, brainstem, or cerebellum. According to their investigations, the deep grey matter structures were involved in 57% of patients, but haemorrhage was seen in more than half of the cases. CT and conventional MR images were normal or showed minimal changes in the early stages. Lesions were initially seen only by diffusion-weighted imaging and were able to show abnormalities in a substantially more extensive distribution of approximately 70% [9].
It could be quite possible that HSV-2 encephalitis presents with only meningeal signs without other typical symptoms, such as vomiting, nausea, altered mental status, and neurological deficits [8]. Although the presence of lymphocytic pleocytosis and increased protein levels with normal glucose levels in CSF accompanied by clinical symptoms and signs are highly in favor of HSV encephalitis, HSV PCR is the gold standard method for the diagnosis of HSV encephalitis [10, 11]. Clinical manifestations and findings on magnetic resonance imaging (MRI) usually complete the diagnosis. In patients with immunosuppression, atypical clinical manifestations require prompt treatment with acyclovir until HSV encephalitis is ruled out [12].
According to positive COVID-19 IgG in this present case and a history of infection highly probable of COVID-19 during the previous three weeks, three differential diagnoses were considered more prominent. Viral or immune-mediated cerebellitis was considered the primary differential diagnosis. Other diagnoses included post-infectious ataxia and infratentorial ADEM. The diagnosis of post-infectious ataxia would be ruled out regarding multiple abnormal signals in the cerebellum. The increased titer of HSV-2 serum IgG and IgM antibodies showed that the infection was not caused by a new infection and was more probable to be caused by the reactivation of the virus. It is possible that the virus was transmitted to the infant through the mother during pregnancy. Although HSV-2 PCR had been reported positive in CSF, this is not mentioned in studies as a common cause of brain involvement for cerebellitis and encephalitis. However, it could result in relapsing aseptic meningitis [13].
CONCLUSION
HSV viruses can lead to dramatic neurological disease of the CNS through primary infection or following virus reactivation. Treatment for HSV with acyclovir and COVID-19 treatment can help patients to recover. Recently, with the widespread SARS-CoV-2 virus infection, children have been affected to a great extent directly by the virus or indirectly through its consequences on the immune system, neurological system, and vital organs [14]. The SARS-CoV-2 virus can reactivate almost all DNA viruses, such as HSV, leading to encephalitis and cerebellitis.
AUTHORS' CONTRIBUTION
EF and VM analyzed and interpreted the patient data regarding the neurologic disease. RA and TL performed follow-ups with the patient and contributed to writing the manuscript. All authors read and approved the final manuscript.
LIST OF ABBREVIATIONS
| MRI | = Magnetic Resonance Imaging |
| HSV-2 | = Herpes Simplex Type 2 |
| PCR | = Polymerase Chain Reaction |
| VZV | = Varicella Virus |
| HHV 6-7-8 | = Human Herpes Virus Type 6-7-8 |
| CMV | = Cytomegalovirus |
| EBV | = Epstein-Barr Virus |
| CSF | = Cerebrospinal Fluid |
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
This case study was accredited by the Ethical Committee of Iran University of Medical Sciences.
HUMAN AND ANIMAL RIGHTS
No animals were used in this research. All procedures performed in studies involving human participants were in accordance with the ethical standards of institutional and/or research committee and with the 1975 Declaration of Helsinki, as revised in 2013.
CONSENT FOR PUBLICATION
Written informed consent was obtained from the parents of the patient for publication of this case report and any accompanying images. A copy of the written consent is available for review by the editor of this journal.
STANDARDS OF REPORTING
CARE guidelines were followed.
AVAILABILITY OF DATA AND MATERIAL
The datasets used during the current case study are in the manuscript.
FUNDING
None.
CONFLICT OF INTEREST
The authors declare no conflict of interest, financial or otherwise.
ACKNOWLEDGEMENTS
Declared none.


