All published articles of this journal are available on ScienceDirect.
Blood Viscosity and its Clinical Implications in Ischemic Stroke and Chronic Heart Failure: Insights from a Case Report
Abstract
Background
Blood viscosity has received increased attention as a potential predictor of ischemic stroke risk, particularly in patients with chronic heart failure (CHF). Despite the importance of this link, there has been a notable paucity of comprehensive research on the subject. Hence, the major goal of this study was to shed light on the potential importance of blood viscosity in individuals with ischemic stroke and CHF.
Case Presentation
An 85-year-old male was presented to the emergency department after three days of gradually decreasing consciousness. His medical history included hypertension and CHF. His Glasgow Coma Scale (GCS) was determined to be E2M5V3, and he displayed evidence of upper motor neuron facial palsy as well as right hemiparesis. The clinical assessment scores, which included the NIHSS and mRS, were 10 and 4, respectively. MRI imaging confirmed the existence of several acute infarctions. Other diagnostic procedures, including an x-ray, revealed cardiomegaly and echocardiographic findings were compatible with grade I diastolic dysfunction. His blood viscosity was 8.19 cP, which was much higher than normal. The patient was diagnosed with ischemic stroke, CHF, and hyperviscosity based on these findings. Despite a small increase in blood viscosity to 8.16 cP after a six-day treatment session, the patient showed significant clinical improvement. Unfortunately, he was readmitted immediately after being discharged and died three days later.
Conclusion
This case demonstrates the importance of blood viscosity in the evaluation and prognosis of patients with ischemic stroke and CHF.
1. INTRODUCTION
Stroke is a leading cause of mortality and disability around the globe [1]. In 2019, the prevalence of stroke was 101.5 million, including 77.2 million ischemic strokes [1]. This high prevalence cannot be separated from other exacerbating conditions, including chronic heart failure (CHF) [2]. CHF is believed to have a role in the prognosis of ischemic stroke since it is associated with pro-thrombotic pathways, pro-inflammatory mechanisms, reduced tissue oxygenation, and hemodynamic abnor malities, all of which may alter stroke outcomes [3]. It is believed that the function of blood viscosity mediates the link between ischemic stroke and CHF.
Blood viscosity is the flow resistance caused by the friction of blood cells flowing through blood vessels at different velocities [4]. Various parameters may influence blood viscosity, including hematocrit, erythrocyte morphology, aggregation, plasma components, fibrinogen, age, smoking habits, diabetes, dyslipidemia, and others [4]. Blood viscosity may characterize the physiological state of blood flow and hyperviscosity, one of the risk factors for ischemic stroke [5]. In addition, individuals with CHF suspected of hypoxemia-induced polycythemia often have a high blood viscosity [6].
The function of blood viscosity in influencing the pathophysiology of ischemic stroke in patients with CHF has the potential to be used as a prognostic biomarker. However, no more research has been conducted on this issue. To illustrate this potential, we presented the importance of blood viscosity examination in a patient with ischemic stroke and CHF. The blood viscosity level was found to be linked to the clinical outcome of the patient. Further, written informed consent was obtained before the study and ethical approval was obtained from the Ethics Committee of the Faculty of Medicine, the University of Indonesia in 23 February 2023 with approval number KET-443/UN2.F1/ETIK/PPM.00.02/2023.
2. CASE REPORT/CASE PRESENTATION
A male, 85 years old, was brought to the emergency department with complaints of decreased consciousness for the previous three days. According to the family, he appeared generally weak, accompanied by slurred speech and a slanted mouth. He could still spontaneously open his eyes, ate and drank, and was communicable. He had no history of head trauma, headache, convulsions, fever, cough, runny nose, nausea, vomiting, or diarrhoea prior to losing consciousness. He had a history of hypertension and CHF, diagnosed one month ago. He usually slept with two pillows and had a history of shortness of breath during light activity. He did not smoke or consume alcohol. He took routine medication of 1x10 mg rivaroxaban, 1x4 mg candesartan, 1x40 mg furosemide, 1x1.25 mg bisoprolol, and 1x500 mg citicoline.
There were vital signs within normal limits. Chest examination revealed bilateral coarse crackles at the bases of the lungs. Other physical examinations were within normal limits. Neurological examination revealed Glasgow Coma Scale (GCS) E2M5V3, upper motor neuron facial palsy, and right hemiparesis. Other neurological examinations were within normal limits. The National Institute of Health Stroke Scale (NIHSS) was 10, and Modified Rankin Scale (mRS) was 4.
Laboratory examination showed anaemia (12.7 gr/dL), leukocytosis (17,950/µL), increased procalcitonin (0.06 ng/mL), increased c-reactive protein (27.5 mg/L), increased d-dimer (1,260 ng/ mL), and increased blood glucose (156 mg/dL). The examination showed electro lytes, liver function, and kidney function within normal limits. Magnetic resonance imaging (MRI) showed multiple acute infarctions in the left pons and midbrain, left thalamus, left parahippocampal gyrus, and left posterior horn of lateral periventricular (Fig. 1). In addition, a chronic lacunar infarction was also found in the left putamen without intracranial bleeding, which corresponds to cerebral small vessel disease Fazekas II and is accompanied by cerebral atrophy with Global Cortical Atrophy scale 3. On chest x-ray examination, cardiomegaly with aortic elongation and calcification was found. Electrocardiographic examination showed patho logical Q waves in leads III and V3, depressed ST, and inverted T in leads V3 to V6.
The patient was diagnosed with ischemic stroke, CHF New York Heart Association (NYHA) class III, coronary artery disease, and hyperviscosity. He was given an initial treatment of heparin 10,000 IU/24 hours, candesartan 1x4 mg, furosemide 1x40 mg, bisoprolol 1x1.25 mg, vitamin B6 2x10 mg, vitamin B12 2x50 mcg, folic acid 2x5 mg, and simvastatin 1x20 mg, and was advised to stop rivaroxaban. After initial treatment, the patient was transferred to the stroke unit for further management.
In the stroke unit, a follow-up laboratory examination showed elevated levels of low-density lipoprotein (132 mg/dL). The examinations of other lipid profiles, blood sugar, and uric acid were within normal limits. Echocardiography examination showed ejection fraction 41.8, tricuspid annular plane systolic excursion 27.6, left atrial volume 31, left ventricular mass index 162, mitral regurgitation vena contracta 0.2. These results showed that the cardiac chamber dimensions were not dilated. Further, concentric left ventricular hypertrophy, segmental hypokinetic, mild mitral regurgitation, decreased left ventricular systolic function, grade I diastolic dysfunction with normal left atrial pressure, good right ventricular systolic function, and no thrombus or effusion were found. Carotid Doppler examination showed right intima–media thickness, arrhythmic pulse, and right vertebral artery stenosis. Transcranial Doppler examination was within normal limits. Viscosity examination using a digital micropapillary showed increased blood viscosity (8.19 cP). To address the identified hyperviscosity, the clinical team focused on managing underlying conditions, such as ischemic stroke and CHF, which were deemed immediate threats to the health of the patient.
After six days, the consciousness of the patient improved. Neurological examination showed GCS E4M6Vx, upper motor neuron facial palsy, and right hemiparesis. Other neurological examinations were within normal limits. There was an improvement in NIHSS to 6 and mRS to 3. Although the clinical condition of the patient improved, a re-examination of the blood viscosity showed that it was still high (8.16 cP). However, considering the clinical improvement, the patient was still discharged with warfarin 1x2 mg, vitamin B6 2x10 mg, vitamin B12 2x50 mcg, folic acid 2x5 mg, and simvastatin 1x20 mg and was asked to come for a check-up every week.
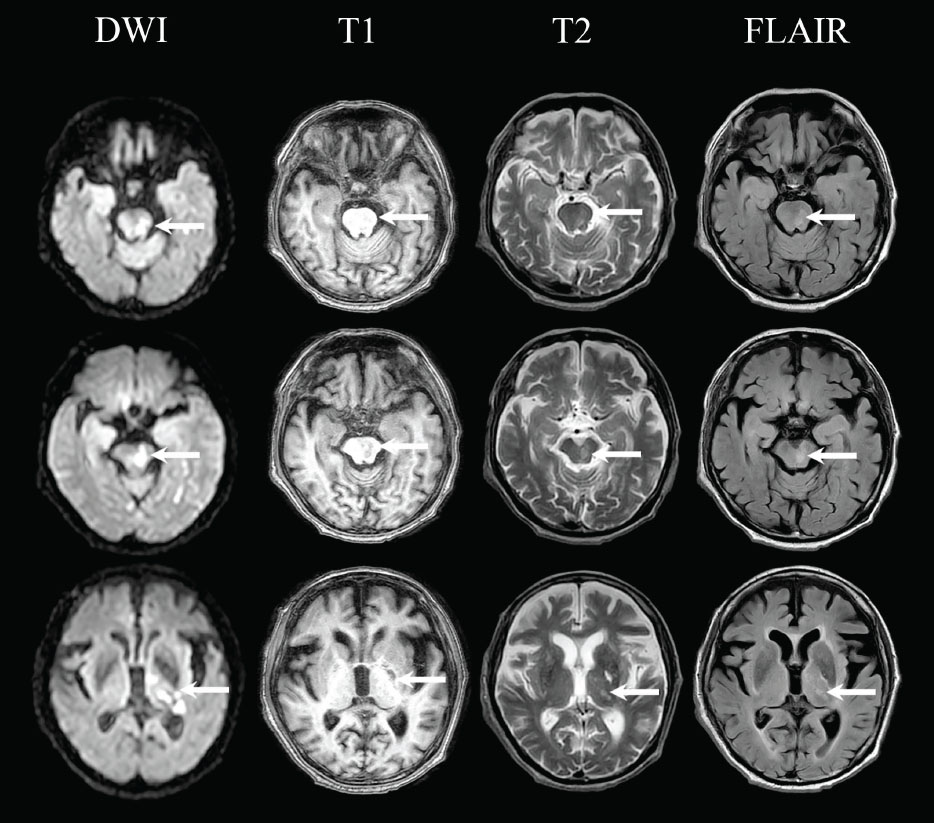
Multiple acute infarctions in the left pons and midbrain, left thalamus, left parahippocampal gyrus, and left posterior horn of lateral periventricular in Diffusion Weighted Imaging (DWI), T1-weighted, T2-weighted, and Fluid Attenuated Inversion Recovery (FLAIR) MRI.
After three days of discharge, the family brought the patient to the emergency department. He was unrespon- sive for 30 minutes. On physical examination, the pulse was not palpable, and respiratory arrest was observed. An electrocardiographic examination showed asystole and the patient was declared dead.
3. DISCUSSION
The examination of blood viscosity in patients plays a role in describing the condition of blood hyperviscosity. This condition can directly indicate increased blood concentration, plasma components, low erythrocyte deformability, and high erythrocyte aggregation [4]. This condition is a risk factor for ischemic stroke [5] and CHF [6], which was crucial in this patient.
Blood viscosity has a relationship with CHF and ischemic stroke. In CHF, there is decreased blood flow, hypoxia, and increased oxidative stress [7]. Hypoxia can induce the formation of erythrocytes, increasing the blood viscosity [7]. In addition, oxidative stress can trigger the formation of reactive oxygen species, resulting in the deformity of erythrocytes [7]. This erythrocyte damage can trigger erythrocyte aggregation [7].
Blood viscosity is also related to ischemic stroke conditions because it can affect blood flow. Increased blood viscosity can be seen in the acute phase of ischemic stroke [8]. Increased blood viscosity can create more significant resistance to blood flow against tissue perfusion, especially in the microvascular area [8]. Increased blood viscosity in acute ischemic stroke conditions is considered to be due to increased hematocrit levels [8]. In addition, high fibrinogen levels in the acute stroke phase can also affect erythrocyte aggregation [8].
The patient, in this case, had hyperviscosity with a blood viscosity of 8.19 cP. Then, the blood viscosity was assessed again and was to be found to be 8.16 cP. The condition of hyperviscosity in patients is influenced by age, hypertension, and hypercoagulation. The patients also found high levels of d-dimer, which could affect the blood viscosity results. This hyperviscosity condition increases the risk of CHF and ischemic stroke because it can lead to hypoxia, increased oxidative stress, and impaired blood flow [4, 8].
Considering the improvement in clinical conditions during treatment, the patient was discharged, even with hyperviscosity. In contrast, hyperviscosity in acute ischemic stroke conditions is associated with poor outcomes, especially with comorbid chronic CHF [9]. In the study of Rasyid et al., 88.6% of patients with acute ischemic stroke had hyperviscosity. About 9.1% of patients had more severe neurologic deficits on the seventh day, and 18.2% had a poor outcome on the 30th day of evaluation [9].
The patient died three days after discharge, most likely influenced by the previous hyperviscosity condition. This worsening clinical outcome may be caused by impaired collateral blood flow due to hyperviscosity, which can expand the infarct area [9]. Therefore, this case conveys that blood viscosity needs to be examined and considered in assessing the prognosis of ischemic stroke patients with CHF. Due to this potential role, blood viscosity has the potential as a prognostic biomarker in ischemic stroke patients with CHF.
The value of this case report rests in its comprehensive description of an older patient with an ischemic stroke and CHF, with a particular focus on the influence of blood viscosity. It provides a unique perspective on the challenges of addressing such diseases in older individuals. This complexity underscores the importance of a comprehensive approach to stroke risk assessment and management in patients with CHF, considering both the potential for increased blood viscosity to contribute to thrombotic stroke risk and the acknowledged risk of cardioembolic events. Future research should aim to delineate the relative contributions of these mechanisms to stroke in the context of CHF, potentially informing targeted interventions that address the specific underlying risks in individual patients.
Nevertheless, the limitations of the study are significant since it relies on a single example, restricting the applicability of its conclusion. Furthermore, while the report emphasizes blood viscosity, it fails to thoroughly investigate other possible variables that may have contributed to the condition of the patient, such as underlying comorbidities or the effects of medication. The absence of a more comprehensive viewpoint may oversimplify the connection between blood viscosity, ischemic stroke, and CHF. To improve generalizability, future research should involve a broader, more diversified sample. Longitudinal investigations may reveal how blood viscosity affects ischemic stroke and CHF development. Additionally, the effects of the different therapies on blood viscosity and patient outcomes should also be studied. Lastly, exploring how blood viscosity interacts with other risk variables, including lifestyle, genetics, and comorbidities, may help us comprehend these complicated disorders.
CONCLUSION
To conclude, hyperviscosity in an ischemic stroke with CHF might impact the prognosis. Moreover, blood viscosity is essential in evaluating the clinical outcome of ischemic stroke patients with CHF.
AUTHORS’ CONTRIBUTIONS
The conceptualization was contributed by A.R., J.R.C., S.H., M.K., R.H., M.Y., and E.W..; methodology was contributed by A.R. and J.R.C.; software was curated by E.W.; validation was provided by A.R., M.K., R.H., and M.Y.; formal analysis was performed by A.R. and J.R.C.; investigation was conducted by A.R. was J.R.C.; resources were contributed by A.R.; data curation was done by A.R., J.R.C., S.H., M.K., R.H., and M.Y.; original draft was prepared by A.R., J.R.C., and E.W.; review and editing were contributed by all authors; visualization was done by J.R.C.; supervision was provided by A.R., S.H., M.K., R.H., and M.Y.; project administration was headed by J.R.C. and E.W.; funding acquisition was carried out by A.R. All authors read and agreed to the published version of the manuscript.
LIST OF ABBREVIATIONS
| CHF | = Chronic Heart Failure |
| GCS | = Glasgow Coma Scale |
| mRS | = Modified Rankin Scale |
ETHICAL STATEMENT
The Ethics Committee of the Faculty of Medicine, the University of Indonesia, approved the report protocols, with protocol number KET-443/UN2.F1/ETIK/PPM.00.02/ 2023.
CONSENT FOR PUBLICATION
Written informed consent for publication was obtained from the patient before the study was published.
STANDARDS OF REPORTING
CARE guidelines were followed.
AVAILABILITY OF DATA AND MATERIALS
All data generated or analyzed during this study are included in this article. Further inquiries can be directed to the corresponding author [A.K].
FUNDING
None.
CONFLICT OF INTEREST
The authors declare no conflict of interest, financial or otherwise.
ACKNOWLEDGEMENTS
Declared none.


