All published articles of this journal are available on ScienceDirect.
Delayed Diagnosis of Post-traumatic Temporal Lobe Meningo Encephalocele: A Rare Case Report of a Child
Abstract
Introduction:
Bone fracture after head trauma is common in children. When a fracture happens in the temporal bone, Cerebrospinal fluid (CSF) might leak and/or the temporal lobe protrude (named encephalocele) as a mass inside the middle ear or mastoid or both.
Case Presentation:
Here, a 10 year old presents with an initial diagnosis of bacterial meningitis. Three years ago after head trauma he had a forgotten bone fracture. Incomplete improvement after primary treatment was achieved. Finally, after seeing a bone fracture on the right roof of the tympani and soft tissue mass in brain High-Resolution Compound Tomography (HRCT),surgical exploration determined the CSF leakage from a right lobe temporal meningo encephalocele. The bone defect was repaired and the patient had complete improvement.
Conclusion:
In this case with forgotten post traumatic temporal bone fracture, temporal bone encephaloceles lead to CSF leakage inside the middle ear cavity and introduce bacterial meningitis. High-Resolution Compound Tomography (HRCT) of the cranial base defined the bone defect. In recent years, Magnetic Resonance Imaging(MRI) has been known as the best method for the diagnosis of brain tissue herniation in the middle ear cavity. Although to differentiate the encephalocele from other masses (e.g. granulation, cholesteatoma, cholesterol granuloma, etc.) inside the middle ear cavity in an MRI is not easy. Surgical multilayered closure of the dura and simultaneous repair of the bone defect is needed.
1. INTRODUCTION
Meningitis is due to inflammation of the meninges which cover and protect the brain and spinal cord. With diverse etiologic factors, H. Influenza Type B (HIB), S. pneumonia, and Meningococcal infection are the most common bacterial etiologies in childhood [1-3]. In the last decade, widespread vaccination of children for bacterial infection has led to changes in the epidemiology of bacterial meningitis [3-5] Several predisposing factors are known in childhood bacterial meningitis (lower age, exposures to pathogens, head trauma, immune deficiency state, ventriculoperitoneal shunts, CSF leakage, neurological defect, bacterial sinusitis, otitis media, pneumonia, sickle cell disease) [4-7]. The most important way for diagnosis is the CSF analysis and culture.
Bacterial meningitis is one of the common causes in admitted Iranian children [6-10] Chamkhaleh et al. reported the epidemiology and outcomes of meningitis among Iranian children during a 10 year period [6] Imaging studies like cranial Ct - scans or MRIs are used for diagnosis of intracranial complications of bacterial meningitis (brain edema, hydrocephalus and infarcts, focal abscess and cerebral herniation) [8]. Determination of the biologic markers in CSF are used for childhood bacterial meningitis. In some studies, the diagnostic CSF level for CRP, procalcitonin, and ferritin. Interleukin in Iranian children with meningitis was published [7, 9, 10].
Encephalocele is a kind of neural tube defect. Post-traumatic skull fracture is common in children. After a fracture, brain tissue herniates through the Dural defect of the temporal bone. Encephalocele presents as a mass inside the middle ear or mastoid or both. Secondly, CSF leaks and bacterial meningitis are added [9-11] Temporal lobe encephalocele is a rare disease with an incidence rate of 1/3000 to 1/35000(9). Before 2015, only 27 cases of temporal lobe epilepsy with encephaloceles were reported, either as congenital skull base defects or acquired defects (including tumors, traumatic fractures, radiation, and iatrogenic erosions) [10, 11]. First, diagnostic methods and surgical repair of otologic CSF leakage are discussed by Raine et al [12] Surgical management of post-traumatic temporal bone encephaloceles (and CSF leakage) has been studied by many authors [13-.22] Reddy et al reported that an imaging study is needed for CSF rhinorrhea and otorrhea [15]. Indeed, CSF leakage is diagnosed by Beta2-transferrin determination in CSF [16] Most of the time there are delays in the diagnosis of these situations due to nonspecific signs and symptoms [20-22]. Untreated cases could have different side effects such as meningitis, seizures, venous infarction, and intracranial abscess [23, 24]. Jeevan et al. discussed clinical manifestations, etiology and imaging findings, and surgical treatment of 32 cases with temporal encephalocele [25] Also Lundy et al. presented their experience [26] Lobo and Matthew et al. published a systematic review about the surgical management of CSF fistula [21,, 27] Outcomes of surgical treatment of tegmentum dehiscence and temporalis fascia graft presented by Swanson et al. [28].
In this case, a 10-year-old boy presented with bacterial meningitis. He had a history of a forgotten bone fracture 3 years ago. In surgery, a right temporal bone meningoencephalocele was explored which results in CSF leakage. The defect was repaired and the patient showed complete improvement.
2. CASE REPORT
A 10 years boy was referred and admitted to the pediatric intensive care unit (PICU) of our tertiary hospital (Rasul Akram Hospital) in Tehran, Iran. In the second hospital, he had a fever, delirium, and gradual loss of consciousness (LOC); The Glasgow Coma Scale(GCS) calculated (Table 1) was 8 and finally he was intubated. (GCS) is a widely used neurological scoring system for quantifying the level of consciousness following brain injury [29].
Intubation and severe facial swelling may preclude the ability to score eye and verbal components. For these circumstances, the score is frequently noted with a modifier, i.e. “V1t” (t = tube), or “E1c” (c = closed).
One week ago, he had a headache, vertigo, and intermediate coughs. The frontal and bitemporal headache intensified by head bending, just a one-time fever before admission. He received Co-amoxicalve and azithromycin in the out-patient. There was no pain relief from using the drugs and due to the headache worsening and vomiting, he was taken to the clinic for serum therapy.
In the admission day, he had a fever (39/1), pulse rate=114, respiratory rate=17, blood pressure=116/76.
2.1. Neurologic Exams
Normal pupil size with reaction to light, decreased deep tendon reflexes (DTR), without kerning or Brudzinski sign. and downward Babinski reflexes.
2.2. Chest
Course crackles were heard in both lungs. He was self-extubated about four hours after admission to the PICU and was well oxygenated by nasal cannula.
2.3. Previous History
Three years ago, he had severe head trauma and skull fracture during a car accident and was in a coma for 3 months. He gradually improved and could walk and speak after speech therapy, physiotherapy, and occupational therapy.
2.4. Lab Tests
In addition to blood culture and other primary laboratory tests, LP was done. CSF analysis determined bacterial meningitis on admission day in our hospital.
2.5. X-ray Findings
Aspiration pneumonia in chest CT scan. The initial brain CT scan was normal without any contraindication for lumbar puncture.
Brain Ct-Scan defined a bone fracture on the right roof of the tympani and soft tissue density in the middle ear suggestive of cholesteatoma reported on the day of admission (Fig. 1).
| Component | Response | Points |
|---|---|---|
| Eye | Eyes open spontaneously | +4 |
| Eye opening to verbal command | +3 | |
| Eye opening to pain | +2 | |
| No eye opening | +1 | |
| Not testable* | NT | |
| Verbal | - | - |
| Oriented | +5 |
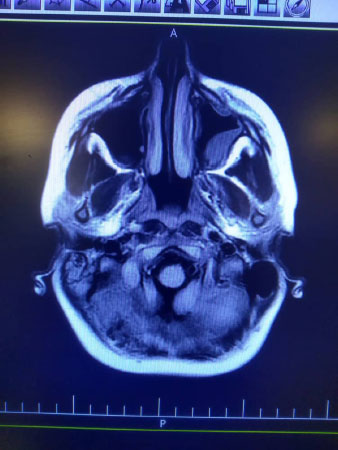
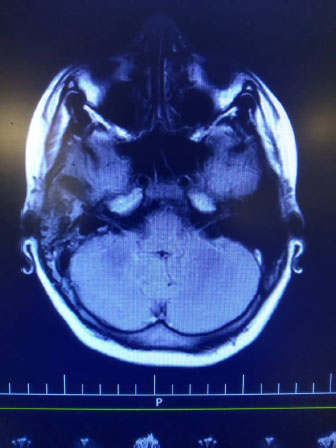
Indeed, high signal intensity in both hemispheres (meningitis), left maxillary and bilateral frontal sinusitis, polyp, and retention cyst in the left maxillary sinus, and right mastoid air cell effusion was reported (Fig. 2).
Due to persistent headache and right ear pain, the spiral HRCT of the temporal bone without injection was done.
In spite of mastoid cell opacity, normal audiometry was reported (Fig. 2).
2.6. Treatment
Meningeal dose of Ceftriaxone (100 mg/kg/day) and vancomycin (20mg/kg/q6h) were started. Dexamethasone (0.6 mg/kg/day), Phenytoin (5mg/kg/day/BD), Acyclovir (10mg/kg/ dose/TDS), and pneumococcal vaccine, were added. He was alert on 2nd day and on the3rd day he was transferred to the pediatric ward. After 10 days of treatment, LP was done again which was absolutely normal and his headache was relieved more than before. He was discharged, for further visit by ENT and neurosurgeon. Subsequently, after 2 weeks (second admission) heunderwent a surgical operation by ENT(ear, nose, and throat)service. A craniotomy in the temporal bone on the root of the zygoma was made 5 cm by 5 cm. The dura is elevated from the floor of the middle fossa. The bone defect was found in the anterior medial tegmental tympani, with a meningoencephalocele of the right temporal lobe accompanied by a CSF leak. Surgical repair of the bone defect was done. A piece of bone was placed on the fascia, and extradural fascia was placed on the site of perforation and fixed on the perforated area with the help of fibrin glue to prevent CSF leakage. The bony flap was fixed in place. A mastoid dressing completes the closure. The case was visited 2 and 6 months after surgery without any neurologic deficits and normal hearing.
3. DISCUSSION
This case report was a 10-year-old boy admitted to our center with a final diagnosis of bacterial meningitis and a forgotten post-traumatic bone fracture 3 years ago. Finally, a right temporal bone meningoencephalocele accompanied by CSF leakage was found during his surgical repair. After surgical repair of the bone defect, he improved and was discharged with full improvement, On the follow-up visits, (2 and 6 months) no neurologic sequels were found and he had a normal hearing evaluation.
In our opinion a delayed diagnosis of skull fracture induced CSF leak and probably, bacterial meningitis. It is opposed to one study reported in which the skull fracture (either with CSF leak) was resolved spontaneously within 1 to 2 weeks [14, 18-23] Less common presentations include meningitis and seizures [24-27]. The clinical presentation of these cases is similar to other studies [19, 20, 27, 28]. Similar data was reported in a study conducted by Jeevan et al. (2015) [28] In contrast to Carlson et al.’s report [21,27] the presented case with confirmed temporal bone encephalocele had any persistent otorrhea or conductive hearing loss, before admission. In contrast to other studies meningitis and seizures were presented in our case [18, 17]. Unlike other reports, no soft compressible mass was seen in the mastoid cavity [17] Temporal bone encephaloceles are rare in children and usually occur when brain tissue herniates through a Dural defect of the skull. Due to nonspecific signs and symptoms, delays in the diagnosis are usual. Untreated cases could have different side effects such as meningitis, seizures, venous infarction, and intracranial abscess [7-13]. In recent years, in addition to CT scans, Magnetic Resonance Imaging(MRI) was known as the best method for diagnosis of brain tissue herniation in the middle ear cavity [15]. Other imaging modalities such as radio nucleotide cistern gram study may be helpful [15, 16], and could distinguish the encephalocele from other masses (e.g. granulation, cholesteatoma, cholesterol granuloma, etc.) [15]. Usually, multilayered closure of the dura and simultaneous repair of the bone defect is needed in cases with confirmed encephalocele Soft tissue repairs without bone repair result in recurrence in some cases.
Usually, multilayered closure of the dura and simultaneous repair of the bone defect is needed in cases with confirmed encephalocele. Soft tissue repairs without bone repair result in recurrence in some cases. Temporal bone encephaloceles always are associated with serious complications such as bacterial meningitis and brain abscesses, conductive hearing loss, and chronic middle ear effusion [10, 11]. Encephalocele manifests as a mass or cerebrospinal fluid (CSF) leak in the middle ear or mastoid (or both). It might have occurred either due to congenital skull base defects; or an acquired form [10-12]. In the past 10 years. CSF otorrhea and temporal lobe encephaloceles have been reported more often than before [11, 12].
4. STRENGTHS OF STUDY
The rapid diagnosis was done in our case by extreme use of imaging diagnostic studies (CT scan) and laboratory tests. Successful repair of defects without any sequels was achieved in our case.
Limitations of the study: A retrospective single case report is the limit of the study. The prospective reports for complications of skull fracture in children would be helpful.
CONCLUSION
After the temporal lobe fracture, an encephalocele mass inside the middle ear cavity might have formed and bacterial meningitis due to CSF leakage was the clinical presentation in the case. The differentiation of the encephalocele in the middle ear cavity from other masses was observed. Usually, nonspecific signs and symptoms result in delayed diagnosis of temporal bone encephalocele. Bacterial meningitis, and neurologic sequels (seizures, venous infarction, and intracranial abscess) might have happened. A High-resolution CT scan (axial and coronal) of the cranial base could define the bone defect.
LIST OF ABBREVIATIONS
| CSF | = Cerebrospinal Fluid |
| HIB | = Hemophilus Influenza type B |
| PICU | = Pediatric Intensive Care Unit |
| DTR | = Deep Tendon Reflexes |
| LP | = Lumbar Puncture |
| MRI | = Magnetic Resonance Imaging |
| HRCT | = High-Resolution Compound Tomography |
| CT | = Compound Tomography |
| ENT | = Ear, Nose, and Throat |
| GCS | = Glasgow Coma Score |
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
Not applicable.
HUMAN AND ANIMAL RIGHTS
Not applicable.
CONSENT FOR PUBLICATION
The consent for the publication of personal detailed data was obtained from parents.
STANDARDS OF REPORTING
CARE guidelines were followed.
AVAILABILITY OF DATA AND MATERIAL
The datasets used during the current study are available from the corresponding author upon reasonable request.
All authors confirm the figures, and tables in the above article are original and do not need any permission /copyright holder(s).
FUNDING
This case presentation was funded by the Research Deputy of Iran University of Medical Sciences Faculty of Medicine.
CONFLICT OF INTEREST
The authors declare no conflict of interest in preparing this study.
ACKNOWLEDGEMENTS
The authors would like to thank the Rasul Akram Hospital for their technical assistance. This study was supported by a grant received from Iran University of Medical Sciences, Tehran, Iran.


