All published articles of this journal are available on ScienceDirect.
Post-COVID-19 Sydenham Chorea: A Case Report
Abstract
Background:
Sydenham chorea, or rheumatic chorea, is a movement disorder that is more prevalent among young people, with a mean age at symptom onset between 8 and 9 years. The condition is more common in females. Sydenham chorea is associated with rheumatic fever and is considered the most common cause of acute chorea in children. We believe that the present case is worth reporting since the occurrence of Sydenham chorea as a post-COVID-19 sequela has not been described in Brazil.
Case Presentation:
We report here the case of a 14-year-old girl with symptoms of acute chorea that emerged 15 days after treatment resolution of COVID-19 (SARS-CoV-2 or severe acute respiratory syndrome coronavirus 2). Brain computed tomography (CT) and magnetic resonance imaging scans showed no changes, and the laboratory tests revealed no signs of an active infectious process. In contrast, neurological positron-emission tomography/CT showed mild glycolytic hypometabolism in the bilateral mesial frontal region. Administration of an oral anticonvulsant resulted in a marked improvement in her symptoms.
Conclusion:
Despite major efforts of the scientific community for discovering treatments, preventive methods, mechanisms of action, and possible sequelae of SARS-CoV-2, there is still a long way to go to better understand this devastating pathological agent that has affected the global population.
1. INTRODUCTION
Sydenham chorea (SC) is one of the main diagnostic criteria for rheumatic fever. It is a neurological disorder characterized by involuntary and incessant movements that affect limbs, face, and trunk. These movements are potentiated in stressful situations and improve during sleep. The condition begins with a benign appearance, usually characterized by emotional lability and muscle weakness. Symptoms prog-ressively worsen and are often accompanied by ataxia, dysarthria, hypotonia, and difficulty writing [1]. Neuro-psychiatric manifestations, such as tics and obsessive-compulsive disorder, have also been reported.
In contrast to the other main signs of acute rheumatic fever, such as joint and cardiac manifestations, that occur 1 to 3 weeks after pharyngitis caused by group A beta-hemolytic streptococci, SC is often a late manifestation that occurs 1 to 6 months after infection [2-5]. Sydenham chorea is usually self-limiting and lasts on average 2 to 3 months, although cases with a duration of more than 2 years have been reported. Some studies suggest that the occurrence of SC in childhood or adolescence may predispose affected individuals to psychiatric disorders in adulthood, including obsessive-compulsive disorders, depression, tics, and difficulty concentrating. However, the long-term psychiatric prognosis of SC is still controversial [4-6].
Therefore, we report here the case of a 14-year-old girl with symptoms of acute chorea that emerged 15 days after treatment resolution of COVID-19 (SARS-CoV-2).
2. CASE PRESENTATION
A 14-year-old female patient was referred to the author's clinic for neurological assessment. The girl had an unremarkable history and always enjoyed good health. Fifteen days after undergoing treatment for COVID-19 infection (February 2021), the patient began to show “strange behavior” according to her mother. The mother also reported that, during treatment of the infection, her daughter developed intense headache with cough and expectoration, smell and taste alteration, and a fever of 38.5 degrees Celsius. The COVID-19 test (SARS-CoV-2) was positive, and the girl had not been vaccinated. The patient thus received an oral antibiotic, anti-inflammatory and symptomatic therapy (at her home), and remained oligosymptomatic 10 days after treatment, with satisfactory clinical outcomes, except for a slight persistence of smell and taste alteration. The cardiopulmonary test was normal, and the patient was thus discharged from medical treatment.
Fifteen days after discharge, the patient began to develop uncontrollable involuntary movements of the arms, legs, face, and trunk, emotional changes and weakness upon minimal effort, accompanied by gait ataxia, dysarthria, and muscle hypotonia, which worsened in stressful situations and improved during sleep. After the neurological assessment, the diagnosis of SC was confirmed.
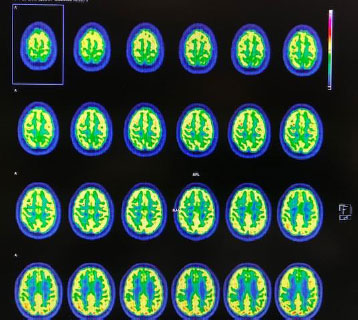
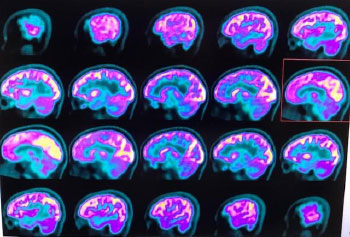
The patient’s brain computed tomography (CT) and magnetic resonance imaging scans showed no abnormalities. Blood laboratory tests were also requested and did not reveal any signs of infectious and inflammatory activity. A neurological positron emission tomography (PET)/CT scan was requested which, after intravenous injection of a radiopharmaceutical tracer (18fluorodeoxyglucose, 18FDG-PET), showed a mild reduction in glucose metabolism in the bilateral mesial frontal region, slightly more evident on the right, as well as in the bilateral frontobasal region (bilateral gyrus rectus and anterior cingulate gyrus). The concentrations of the radiopharmaceutical were preserved in the other cortical regions of the cerebral and cerebellar hemispheres, basal ganglia, and thalamus (Figs. 1 and 2).
The proposed therapy with carbamazepine, an anticonvulsant that is used for several types of SC because of its blocking action of postsynaptic dopamine receptors, resulted in a general improvement in all symptoms one week after the beginning of treatment.
3. DISCUSSION
A wide range of neuronal syndromes, including encephalopathies, brain disorders, acute disseminated encephalomyelitis with hemorrhage and necrosis, transverse myelitis, Guillain-Barré syndrome, and cognitive decline, have been associated with COVID-19 [7-10]. Recently, a multi-system inflammatory syndrome in children has emerged, which shares clinical and laboratory characteristics with Kawasaki disease. These cases usually occur days or weeks after SARS-CoV-2 infection and are called Kawasaki-like syndrome [11-13].
This variety of manifestations points to different underlying physiological mechanisms induced by infection with SARS-CoV-2. In addition to direct viral damage, post-infection inflammation, the production of antineuronal autoantibodies, vasculitis, cytokine-related hyperinflammation, and brain complications of hypoxia and coagulopathy have been discussed [6, 14, 15]. In our case, brain magnetic reso-nance imaging did not reveal any sign of demyelination or vasculitis lesions; in addition, the lack of a clear regional 18FDG-PET pattern ruled out active encephalitis.
The hyperinflammation described is related to an increase in proinflammatory cytokines (tumor necrosis factor) and interleukins (IL), such as IL-6, as a result of an increase in the number of monocytes and macrophages. This process is called “cytokine storm”, which is added to the difficulty in eliminating SARS-CoV-2 due to the induced apoptosis of endothelial and pulmonary epithelial cells [16-18].
In contrast, we do not have a valid explanation for the cortical dysfunction that leads to cerebral hypometabolism and the clinical signs observed. Similar to septic encephalopathy after bacterial infections, a systemic immune response triggering an inflammatory reaction within the brain that can disrupt the blood-brain barrier, impair astrocyte clearance at synapses, and disturb microcirculation, may be suggested [15]. In the present case, cortical hypometabolism may be the remnant of an inflammatory process triggered by a systemic immune response. The fact that SARS-CoV-2 is able to replicate in neuronal cells and infect human brain organoids highlights the neurotropism of this viral agent.
Furthermore, the high rate of smell and taste alteration found in some studies would be in line with the hypothesis of neuroinvasion along olfactory or gustatory fibers. On the other hand, the loss of smell and taste may have been caused by the dysfunction of supporting cells of the olfactory epithelium, regardless of neural involvement [19-25].
Other mechanisms of action must have been responsible for the signs and symptoms of our patient that occurred during her healthy phase of adolescence. A possible explanation could be given, as in other studies, with our result obtained by the images evidenced in the neurological PET/CT, as submitted by the patient [6, 14, 15, 20].
Finally, we understand that the association between SC and SARS-CoV-2 is a recent finding that has not yet been reported in Brazil. We, therefore, highlight the importance of the clinical and radiological alterations reported by the patient, as well as of studies like the present one [26].
4. CONCLUSION
Despite major efforts of the scientific community in discovering treatments, preventive methods, mechanisms of action, and possible sequelae of SARS-CoV-2, there is still a long way to go to better understand this devastating pathological agent that has affected the global population. The characteristics of the present case, together with the fact that knowledge about the possible short-, medium- and long-term consequences of infection with SARS-CoV-2 for global health is still scarce, have led the authors to conclude that it is an important case to be reported.
LIST OF ABBREVIATIONS
| CT | = Computed Tomography |
| SC | = Sydenham Chorea |
| PET | = Positron Emission Tomography |
| IL | = Interleukins |
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
Not applicable.
HUMAN AND ANIMAL RIGHTS
Not applicable.
CONSENT FOR PUBLICATION
Patient’s consent to report this case has been obtained on the condition that all details that would enable any reader to identify the person will be omitted.
STANDARD OF REPORTING
Care guidelines have been followed in this study.
AVAILABILITY OF DATA AND MATERIALS
The data supporting the findings of the article is recorded in the medical records of the treating service (Instituto de Neurosciências, Newclin Clínica Médica especializada de Sorocaba, São Paulo, Brazil), and is confidential.
AVAILABILITY OF DATA AND MATERIALS
The data supporting the findings of the article is recorded in the medical records of the treating service (Instituto de Neurosciências, Newclin Clínica Médica especializada de Sorocaba, São Paulo, Brazil), and is confidential.
FUNDING
None.
CONFLICT OF INTEREST
The authors declare no conflict of interest, financial or otherwise.
ACKNOWLEDGEMENTS
Declared none.


