All published articles of this journal are available on ScienceDirect.
Electroencephalography Findings in Traumatic Brain Injury
Abstract
Traumatic Brain Injury (TBI) or also known as a head injury is one of the leading causes of death among young people and is also one of the health problems defined as impact, penetration, and rapid movement of the brain within the skull that can result in changes in mental status and is often referred to as the silent epidemic.
Head injuries can also result in electrophysiological abnormalities seen on electroencephalography (EEG) recordings. Electroencephalography was the first clinical neurodiagnostic assessment to reveal abnormal brain function after a head injury. For detecting brain injury, EEG may be more sensitive than clinical neurologic examination.
In general conditions, electroencephalography is performed on patients with moderate to severe head injuries to provide information about the severity of the head injury, and establish a diagnosis of non-convulsive status epilepticus. This diagnosis often follows head injuries because patients with loss of consciousness are difficult to identify without an EEG examination. This also helps determine a prognosis for recovery and the likelihood of post-traumatic seizures.
Electroencephalography features in head injuries depend on the severity of the injury and the location of the head itself. Electroencephalography after head injury shows slowing of the posterior dominant rhythm and increasing diffuse theta slowing, which returns to normal within hours or may subside more slowly over several weeks. Electroencephalography changes are not the same in each individual, due to differences in the severity of head injuries. It is important to know that there is no clear or specific EEG for mild traumatic brain injury.
1. INTRODUCTION
Head injury is a change in brain function or there is evidence of pathology in the brain caused by external mechanical forces. Head injuries can be caused by mechanical trauma to the head, either directly or indirectly, which causes impaired neurological function in the form of temporary or permanent physical, cognitive, and psychosocial functioning disorders [1]. Head injuries are categorized based on clinical post-traumatic amnesia (PTA) and/or disturbance of consciousness (alteration of consciousness (AOC) or loss of consciousness (LOC) [2].
Based on data obtained from the Centers for Disease Control and Prevention (CDC), as many as 1.7 million people experience head injuries each year in the United States. Langlois et al found that more than 1.1 million people in the United States suffer head injuries each year. The incidence of head injury in Europe in 2010 was 500 per 100,000 population [3]. Based on Riskesdas data in 2013, the highest prevalence of injury in Indonesia nationally was found in South Sulawesi with falls and motorcycle accidents being the most common causes of injury. According to the distribution of age groups, head injuries are more common in patients of productive age [4]. Epidemiological data on head injuries at Dr. Wahidin Sudirohusodo Hospitals in Makassar showed an increase in the number of head injuries in 2005 to 1078 in 2007 [5].
1.1. The Role of Electroencephalography in Traumatic Brain Injury
Traumatic brain injury can be classified based on the degree of severity into mild, moderate, and severe. Several parameters such as the presence of construction disorders, knowing awareness, duration of post-traumatic mental status changes, and Glasgow Coma Scale (GCS) scores are used to determine several classifications. The division of severity can be seen in Table 1 regarding the classification of the severity of head injuries.
| Criteria | Mild | Moderate | Severe |
|---|---|---|---|
| Imaging | Normal | Normal or Abnormal | |
| Duration of loss of consciousness | 0 – 30 minutes | > 30 minutes to < 24 hours | > 24 hours |
| Duration of altered mental state | Up to 24 hours | > 24 hours, severity based on other criteria | |
| Post traumatic amnesia | 0-1 day | > 1 to < 7 days | > 7 days |
| GCS | 13-15 | 9-12 | < 9 |
Patients with traumatic brain injuries require serious monitoring of clinical symptoms that arise to predict the possible worsening of the condition that can develop into post-traumatic seizures. The Glasgow Coma Scale (GCS) plays an important role in determining clinical decisions for brain head injury patients, one of which is whether or not the head injury patient requires a neuroimaging examination in the form of a CT scan and other further examinations. The Glasgow coma scale is one component that is used as a treatment reference, and the basis for making general clinical decisions for head injury patients [5, 7].
In general conditions, electroencephalography is performed on patients with moderate to severe head injuries to provide information about the severity of the head injury, and establish a diagnosis of non-convulsive status epilepticus. This diagnosis often follows head injuries because patients with loss of consciousness are difficult to identify without an EEG examination. This also helps determine the prognosis for recovery and the likelihood of posttraumatic seizures.
In addition, it is often requested when patients with post-traumatic syndromes are being evaluated at a later stage, in the hope that it will indicate whether nonspecific symptoms have an organic etiology. No specific EEG abnormalities developed after head injury. The presence of local abnormalities is often of particular concern because it may indicate the presence of an intracerebral, subdural, or extradural hematoma. Unfortunately, there is no reliable way to differentiate electroencephalographically between surgically repairable intracranial lesions, such as subdural hematomas, and pathologies that do not require operative treatment. Under such circumstances, judicious use of imaging procedures facilitates rational management of individual patients [8-10].
The EEG features of a head injury depend on the severity of the injury and the location of the head itself [10]. The EEG after a head injury shows a slowing posterior dominant rhythm and increased diffuse theta slowing that may return to normal within hours or may disappear more slowly over several weeks. Other abnormalities that may be present after a head injury include localized or generalized depression of normal activity, focal or diffuse slow-wave disturbance, especially in the temporal region in adults, and spike or paroxysmal seizures. These changes may develop progressively over time if a serial recording is performed. Focal abnormalities may be directly related to local injury but can also occur as sequelae to complications of cerebral trauma, such as ischemia or edema. It is important to know that there is no clear or specific EEG for mild traumatic injury [8, 9].
1.2. EEG changes in Post-head Injury Patients [11]:
- Post-acute injury
Electroencephalography Immediately after injury, there is epileptiform activity (high amplitude sharp waves or high frequency) followed by diffused cortical activity usually lasting 1–2 minutes, followed by a diffused slowing EEG and returning to normal in 10 minutes to 1 hour. The EEG most often shows decreased alpha frequencies, with increases in theta and delta [11].
- Electroencephalography after sub-acute injury
Several weeks to months after injury there is an increase in the frequency of 1-2 Hz in the posterior alpha rhythm.
- Electroencephalography after chronic injury
Patients with the post-injury syndrome will show an increased frequency in the delta (1.5 – 5 Hz) and a decrease in the alpha frequency (8.5 – 12).
Post-traumatic seizures (PTS) and post-traumatic epilepsy (PTE) are complications of traumatic brain injury. A post-traumatic seizure is a single or recurrent seizure that occurs less than 1 week after a head injury and is provoked by a lesion in the brain due to the head injury. Post-traumatic epilepsy is one or more unprovoked seizures that occur more than 1 week after the head injury. The incidence of PTE ranges from 2 to 16%. Electroencephalography examination is needed to establish a diagnosis of seizures and the severity of the head injury, to identify the depth of loss of consciousness, and determine the prognosis (survival/outcome) of a head injury [9].
The main use of the EEG is to diagnose non-convulsive status epilepticus, which may follow a head injury. Research shows that in 22% of head injuries, clinical manifestations of convulsive and non-convulsive seizures can occur (Vespa et al, 1999, Aquino et al, 2017). A 2012 case study showed EEG features with severe deceleration and suppression of the delta waves associated with worsening outcomes at 3-6 months in patients with a head injury. In a meta-analysis of the ability of the EEG as a prognostic factor in 44 studies of severe head injury by excluding obvious local lesions, decompression craniotomy, or the presence of subdural and extradural fluid buildup, the EEG results of no bilateral potential somatosensory evoke were associated with a worsening outcome in 99.5%. patient [10].
The EEG images that can be found are [9]:
- Epileptiform activity
- Diffused slowdown
- More severe patterns such as periodic patterns, burst suppression, and electro cerebral inactivity.
- Less common patterns such as alpha coma, theta coma, and spindle coma.
1.3. Epileptiform Activity
Focal epileptiform features predispose to focal seizure onset. Epileptiform eruptions can be isolated or in the form of a series of brief flashes. The most common epileptiform triggers are spikes and sharp waves [12].
The focal epileptiform wave spectrum (Fig. 1) can be [13]:
(1) Spikes
(2) Sharp Waves
(3) Multiple Spike Complexes (Polyspike Complexes)
(4) Spike-Wave Complexes (Spike and Slow Wave Complexes)
(5) Polyspike Wove Complexes (Multiple Spike and Slow Wave Complexes)
1.4. Epileptiform abnormal course Committee on the terminology of The International Federation of Societies for Electroencephalography and Clinical Neurophysiology (IFSECN) July 2016
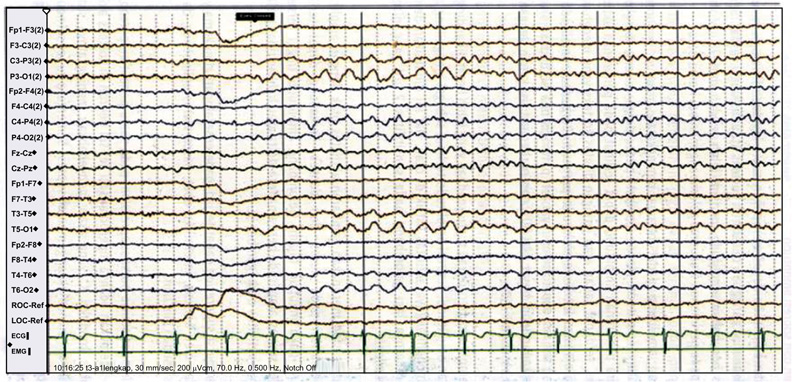
The EEG image of a 78-year-old patient following a head injury shows a mild diffused slowing of the background rhythm and sharp waves in the left anterior region Fig. (2) [12].

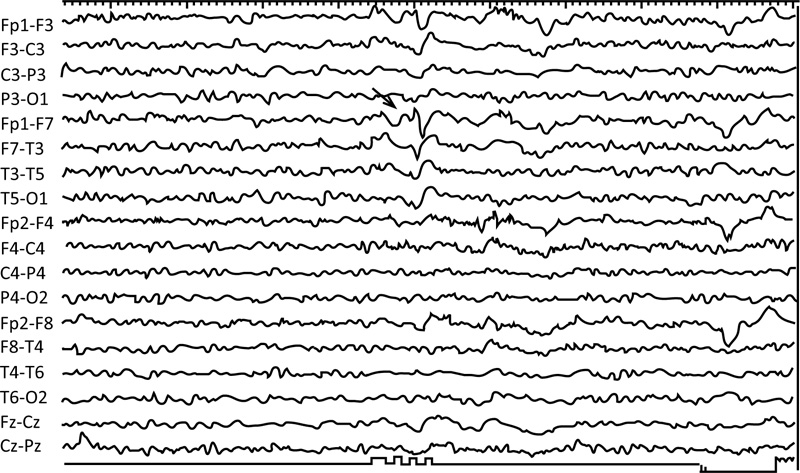

The EEG images of a 30-year-old suspected patient with post-head injury focal epilepsy. (Fig. 3) shows a burst of generalized and bilateral synchronous 3 Hz spike-wave activity in the linked ear referential montage (A1-A2). Low-frequency filter 0.5 Hz, high-frequency filter 70 Hz [14].
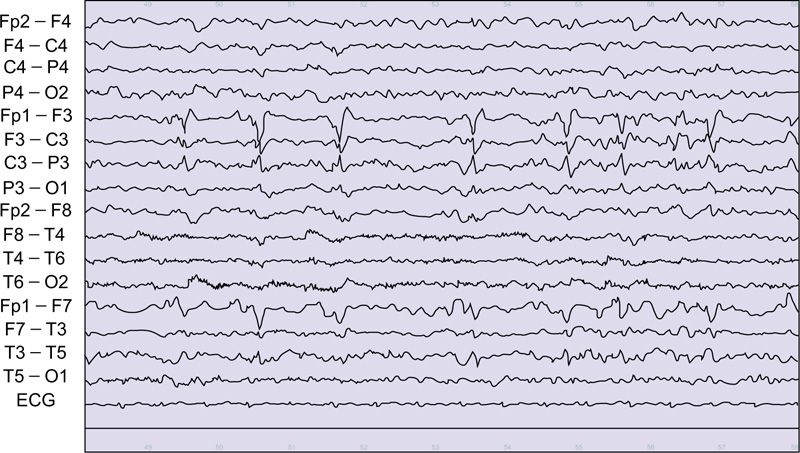
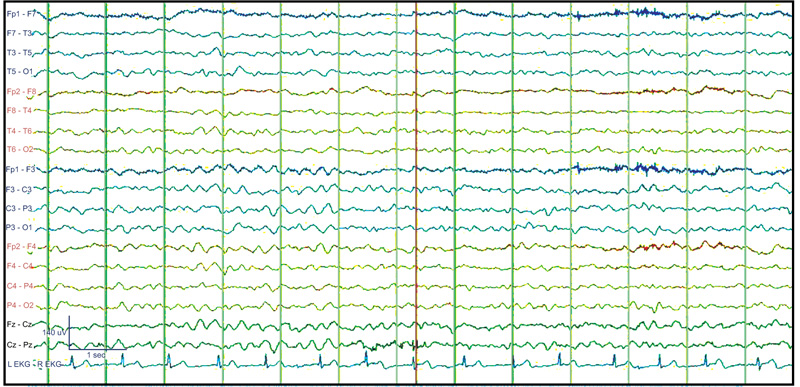
Lateralized periodic discharges (LPD; previously known as periodic lateralized epileptiform discharges or PLEDs) can occur in a variety of conditions, in which the patient is fully conscious, such as focal cortical dysplasia, or presents with decreased consciousness to the level of coma. (Comma-LPD). Like other EEG patterns, LPD can be found in a variety of etiologies with cortical pathology (e.g. encephalitis, stroke, subarachnoid hemorrhage, head injury, tumor, cysticercosis, and intoxication) or subcortical pathology. There is a long debate as to whether they represent an ictal, interictal, or semi ictal pattern. Neuroimaging studies (PET and SPECT) and post-treatment improvement have debated the ictal nature of some forms of LPD (Fig. 4) [15].
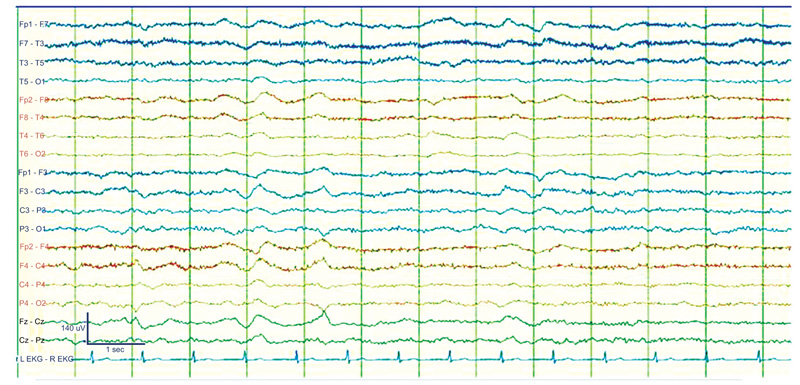
Figure 5 shows an EEG recording of a 3-month-old Abusive Head Trauma victim with seizures.
(https://doi.org/10.1016/j.yebeh.2015.05.005)
(https://thejns.org/pediatrics/view/journals/j-neurosurg-pediatr/16/2/article-p167.xml)

(https://doi.org/10.13004/kjnt.2013.9.2.64)
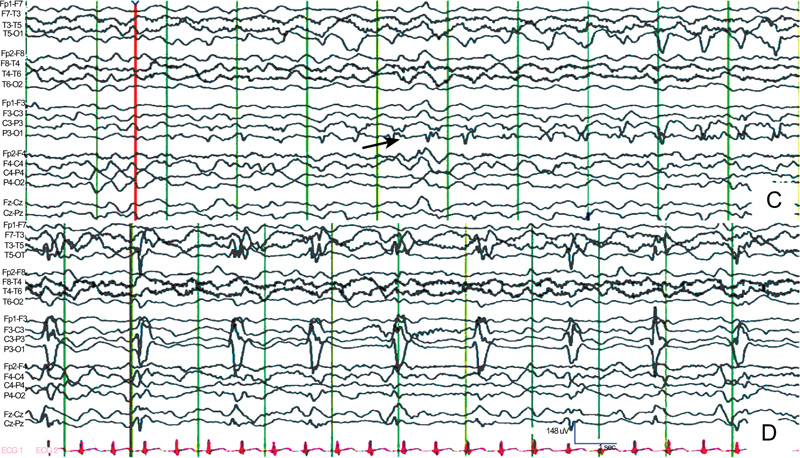
C: EEG recording shows an ictal discharge developing at about 2 Hz (arrow) arising from the left occipital region. D: Seizures continued 74 seconds later. There is a high-amplitude 1-Hz spike reflected in most of the left hemisphere [16].
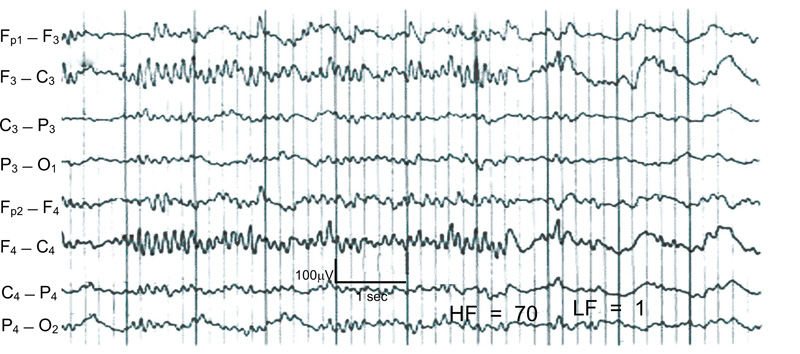
A case of nonconvulsive status epilepticus in an 80-year-old woman. She showed repeated episodes of facial twitching and nystagmus. A: Computed tomography demonstrating acute subdural hematoma in the right fronto-temporo-parietal area with a hemorrhagic contusion.
B: Typical EEG finding of nonconvulsive seizure. Ictal activities are seen on the right fronto-temporal (F8-T4, T4-6) and right fronto-parietal (F4-C4, C4-P4) area. Note that during the ictal period, there is no artifact from muscle contraction in other surface electrodes, which is normally accompanied by convulsive seizures (Fig. 6) [17].
2. Slow Wave
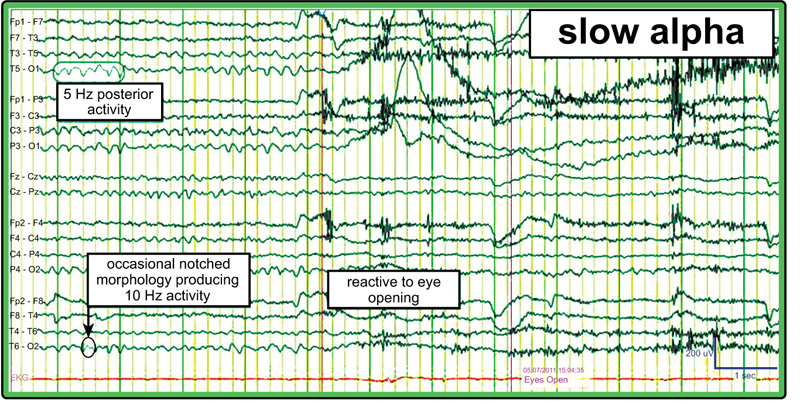
Background posterior dominant rhythm slowing is a nonspecific finding, suggesting a mild diffuse encephalopathy (cerebral dysfunction). This is usually in the theta range between 4 and 7.5 Hz. It can be chronic due to an underlying neurocognitive disorder such as dementia or traumatic brain injury, or it may be acute in hospitalized patients receiving sedatives or with toxic metabolic disorders. The sub-harmonic variant of the normal alpha rhythm can appear in half of the normal posterior dominant rhythm (PDR) frequency, which usually has a notch in appearance and disappears with the eyes open. PDR attenuation can also be viewed as a normal low-voltage variant, in which case the principles underpinning the normal background include reactivity, variability, and an anterior to posterior gradient with a faster anterior frequency [18].
Slow alpha image with a notch and disappears with the eyes open [18, 19].
The slow-wave characteristic is a wave with a frequency below 8 Hz in adults at some electrodes or throughout the recording. Deceleration can be synchronous / asynchronous, intermittent / continuous, focal / common. The deceleration can indicate a normal or abnormal condition. The amplitude is higher than the background, asymmetry, and dominance in one region in Theta rhythm that looks irregular and continuous. Also, the frequency not being influenced by eyes opening can indicate a disorder caused by structural lesions in the brain or mild encephalopathy. If the encephalopathy gets worse, the frequency will decrease to delta (Fig. 7) [20].
A deceleration in the form of delta rhythm has a more significant meaning than theta rhythm. The delta rhythm, when seen in the frontal region, regular, bilateral synchronous, intermittent (frontal intermittent rhythmic delta activity = FIRDA) with a slow background can be a physiological state of sleep or hyperventilation. However, FIRDA can also be found in several pathological conditions, including midline intracranial lesions, increased intracranial pressure, and metabolic encephalopathy [20].
The regular and intermittent delta rhythms seen in the temporal (temporal intermittent rhythmic delta activity = TIRDA) are usually indicative of an abnormality. Temporal intermittent rhythmic delta activity in addition to being a focus of epilepsy, especially when found shortly after the patient has a clinical seizure, can also be an indication of a structural lesion in the brain. Suspicion of a structural lesion in the brain increases when an irregular delta rhythm (polymorphic delta activity = PDA) is seen on one side [20].
The occipital region can also show regular and intermittent delta rhythms called OIRDA or PIRDA (posterior intermittent rhythmic delta activity). Occipital intermittent rhythmic delta activity can also be found in head trauma, migraine, and toxic encephalopathy but is very rare (Fig. 8) [20].
(https://eegatlas-online.com/index.php/en/alphabetical-index/alpha-rhythm-guest)
(https://www.learningeeg.com/slowing-and-other-non-epileptiform-abnormalities#)
(https://www.medscape.com/answers/1140075-177587/what-is-intermittent-slowing-on-eeg)
Describes moderate to severe diffuse encephalopathy, from a variety of causes including a post-ictal state following a generalized seizure, strong sedative effects such as propofol or midazolam, or may reflect significant developmental static encephalopathy or extensive head injuries such as anoxic brain injury or diffuse axonal injury (Fig. 9) [18].
This is a typical example of a general intermittent slowdown. This abnormality of the EEG waveform is very nonspecific and can be caused by a variety of causes including head injury. It is most commonly seen as polymorphic delta and/or theta activity which in adults is most often frontal dominant (Fig. 10) [18].
Electroencephalography activity 8 to 13 Hz that is incessant and unresponsive to eyes opening or other stimuli is called an alpha coma. This activity differs in appearance from the alpha rhythm (normal background activity) in its lack of reactivity and spatial distribution. Alpha coma may be monorhythmic or diffused or may have anterior or posterior accentuation. Only small fluctuations in amplitude occur, and minimal or no reactivity to external stimuli can be elicited [18].
Electroencephalography activity 8 to 13 Hz that is incessant and unresponsive to eyes opening or other stimuli is called an alpha coma. This activity differs in appearance from the alpha rhythm (normal background activity) in its lack of reactivity and spatial distribution. (Fig. 11) Alpha coma may be monorhythmic or diffused or may have anterior or posterior accentuation. Only small fluctuations in amplitude occur, and minimal or no reactivity to external stimuli can be elicited [18].
(https://www.medscape.com/answers/1140075-177590/what-is-an-alpha-coma-on-eeg)
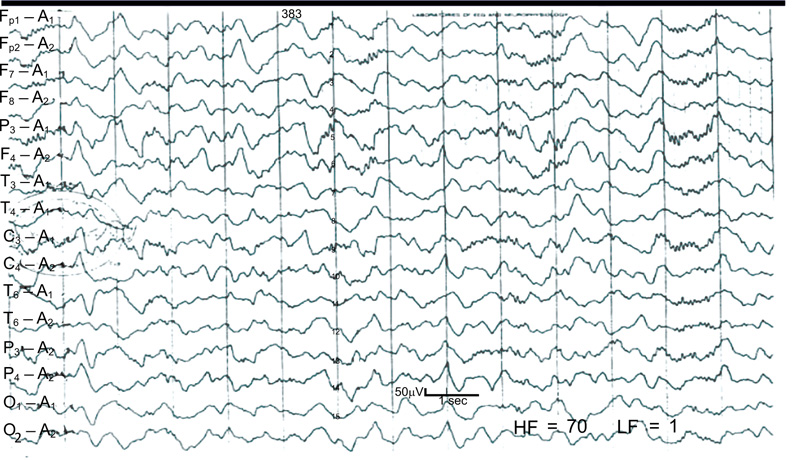
The spindles coma is similar in appearance and implication but consists of monorhythmic 11 to 14 Hz activity, occurring paroxysmal on a delta background without reactivity. Such a pattern is frequently seen in anoxia, head injury, and diffuse cerebral disorders. Prognosis usually depends on the underlying etiology, although reactivity signals a better prognosis than no reactivity on this EEG [18].
This 14-year-old man patient suffered a closed head injury. At the time of the EEG (3 days after the injury) the patient was comatose, but respirations were spontaneous and he responded appropriately to painful stimulation. There is a generalized high-voltage delta activity with the sleep spindle superimposed. The spindles are more widespread than normal sleep spindles, although they are similar morphology. The patient gradually improved to normal neurological function (Fig. 12) [18].
2.1. Amplitude Change and Suppression
Changes in amplitude can be a physiological state or an indication of a disturbance throughout the cortex or temporary dysfunction. Physiological changes in amplitude to be lower than 20 µV can be seen wave frequency changes to a beta rhythm. The change in amplitude can either be an increase or a decrease. An increase in amplitude can be seen in cranial bone defects, while a decrease in amplitude can be seen in decreased activity in the cortex, where fluid or tissue over the cortex blocks the transmission of electrical activity to the recording electrodes. The difference in the amplitude of the right and left sides can have a pathological meaning if the difference between the left and the right is greater than 50% and the difference between the right and left sides is greater than 35% [20].
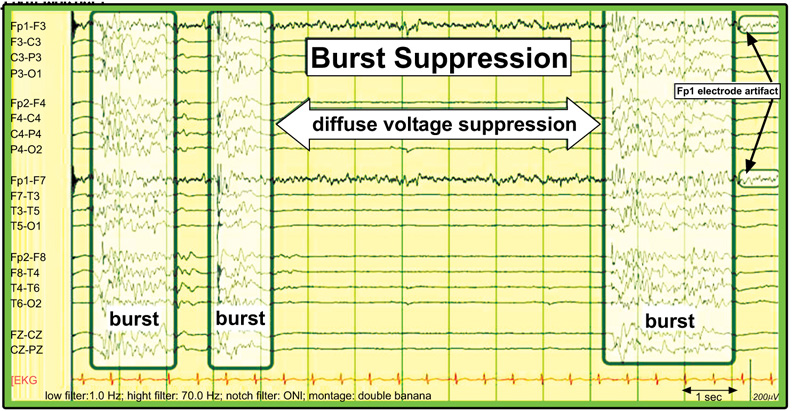
Amplitude suppression is a more severe condition than a decrease in amplitude. Amplitude suppression is seen as a disappearance or near-absence of electrical activity in the brain. This is evidenced by the no longer visible waves at a sensitivity of 2 µV which is called electrical silence. Burst-suppression is a picture of high-amplitude brain activity that varies in duration and form. Sometimes you can see spikes or sharp waves. The high-amplitude waves are followed by suppression of the theta-delta rhythmic amplitude or even the absence of brain activity. The picture of burst suppression can occur in encephalopathy or coma caused by anoxia, drug intoxication, post-status epilepticus, under anesthetic effects, and others. Pathophysiology of burst-suppression, in an animal experimental study, found hyperpolarization of neuron cells in the cortex due to increased K conduction which will cause an increase in the GABAergic system which shows a suppression picture. Meanwhile, neuron cells in the thalamocortical are still sending electrical signals that are caught as bursts [20].
(https://eegatlas-online.com/index.php/en/alphabetical-index/burst-suppression-guest)

(https://eegatlas-online.com/index.php/en/alphabetical-index/breach-artifact-guest)
The burst suppression pattern was originally described as a pattern found in deep anesthesia but is now recognized as a marker of poor prognosis in cerebral hypoxia/anoxia after cardiopulmonary arrest. Burst suppression patterns can also be found in various etiologies (structural such as severe trauma, toxic, and metabolic) or during hypothermia. The pattern consists of periodic high-amplitude, sharply contoured waveforms, including spikes and polyspikes, sometimes with stacking or salvos of spikes alternating with periods of severe or even isoelectric suppression. The trigger lasts from less than one second to 10 seconds or more, and the suppression period may be from one second to 10 seconds or longer (Fig. 13) [16].
Breach Artifact is a high-voltage rapid activity in the area of the skull bone defect. The skull is a filter of high frequencies and voltages especially beta, spike, and sharp rhythms that occur only in skull defects. Better identified with a bipolar montage. Clinical significance: signs of bony abnormalities, not EEG abnormalities (Fig. 14) [19].
The role of EEG in head injury [11]:
1. Diagnostic)
The role of EEG in diagnostics is aimed at detecting seizure activity. Electroencephalography mainly a role in the diagnosis of non-convulsive epilepsy, where clinically the seizures are non-specific, such as anorexia, and aphasia. Mutism, amnesia, aggression, confusion, facial twitching, blinking, shaking, and nystagmus. If the patient is in a state of loss of consciousness at the time of the head injury, the seizures are difficult to identify and require EEG monitoring to determine the severity of the trauma and the depth of the loss of consciousness.
2. Prognosis
Electroencephalography monitoring is also needed to see the outcome of head injury patients. Changes in a wave pattern (alpha, theta, and spindle coma) and lack of reactivity to pain and sound stimuli suggest a poor prognosis of severe head trauma.
CONCLUSION
Traumatic brain injury is one of the leading causes of death among young people and is one of the health problems defined as impact, penetration, and rapid movement of the brain within the skull [7]. Electroencephalography features in head injury depend on the severity of the injury and the location of the head itself. Electroencephalography after head injury shows a slowing of the posterior dominant rhythm and an increase in diffuse theta slowing, which returns to normal within hours or may disappear more slowly over several weeks. Electroencephalography changes are not the same in each individual, due to differences in the severity of the head injury. It is important to know that there is no clear or specific EEG for mild traumatic brain injury [11].
LIST OF ABBREVIATIONS
| TBI | = Traumatic Brain Injury |
| EEG | = Electroencephalography |
| GCS | = Glasgow Coma Scale |
| PTS | = Post-traumatic Seizures |
| PTE | = post-traumatic Epilepsy |
CONSENT FOR PUBLICATION
Not applicable.
FUNDING
None.
CONFLICT OF INTEREST
The authors declare no conflict of interest.
ACKNOWLEDGEMENTS
Declared none.


