Leukocyte Count and Neutrophil-To-Lymphocyte Ratio as Simple Hematologic Predictors of Stroke Severity and Functional Outcome in Acute Ischemic Stroke Patients
Abstract
Background:
It has long been recognized that inflammation plays a critical role in the pathogenesis of ischemic stroke. However, whether leukocyte count and neutrophil-to-lymphocyte ratio are related to stroke severity and functional outcome is uncertain.
Objective:
This clinical study aimed to evaluate the association of leukocyte count and neutrophil-to-lymphocyte ratio with stroke severity and functional outcome in acute ischemic stroke patients.
Methods:
This hospital-based, retrospective observational study included 112 subjects with acute ischemic stroke. All subjects had their demographic, clinical, and laboratory data obtained. The leukocyte count and neutrophil-to-lymphocyte ratio were evaluated by stroke severity on admission and 3-month functional outcome. The severity of stroke at admission was measured using the National Institutes of Health Stroke Scale (NIHSS), whereas the Barthel Index was used to measure 3-month functional outcome (BI). We conducted a regression analysis, adjusting for any confounding variables.
Results:
Higher leukocyte count was significantly associated with increased risk of stroke severity (odds ratio [OR] 1.391, 95% confidence intervals [CI], 1.121-1.725, p: 0.003) and unfavorable functional outcome (OR 1.434, 95% CI, 1.068-1.925, p: 0.017). Higher neutrophil-to-lymphocyte ratio was not significantly associated with increased risk of stroke severity (OR 1.181, 95% CI, 0.947-1.474, p: 0.140) and unfavorable functional outcome (OR 1.246, 95% CI, 0.905-1.716, p: 0.177).
Conclusion:
Our study indicates that leukocyte count is an independent predictor of stroke severity on admission and unfavorable functional outcome.
1. INTRODUCTION
A stroke is a neurological deficit classically defined as an acute focal injury to the central nervous system caused by a vascular cause, such as cerebral infarction, intracerebral hemorrhage, or subarachnoid hemorrhage. It is a leading cause of disability and death worldwide. Ischemic stroke refers to an episode of neurological impairment induced by a focal cerebral, spinal, or retinal infarction [1]. After ischemic heart disease, stroke is the second leading cause of mortality worldwide. Additionally, stroke was the second largest cause of disability-adjusted life years globally. Ischemic strokes accounted for 84.4% (82.1–86.4) of all strokes [2].
Inflammation is a crucial factor in the pathophysiology of ischemic stroke, with a complex process. Both pro-inflammatory and anti-inflammatory mediators have a role in the pathophysiology of ischemic stroke, and an imbalance between the two results in inflammation. Early leukocytosis is associated with the volume of infarcted tissue in patients with acute ischemic stroke [3]. Neutrophils are the first leukocyte subtype recruited to ischemic brain injury; lymphocytes were recruited following neutrophils [4-7].
Leukocyte counts and ratios (for example, the neutrophil-to-lymphocyte ratio) in peripheral blood have been shown to be good predictors of outcome [8, 9]. Prognosis assessment of acute ischemic stroke patients sooner and accurately may minimize the consequences of ischemic stroke. As a result, it is critical to identify novel predictors capable of predicting stroke severity and functional outcome in patients with acute ischemic stroke. This clinical study aimed to evaluate the association of leukocyte count and neutrophil-to-lymphocyte ratio with stroke severity and functional outcome in acute ischemic stroke patients.
2. MATERIALS AND METHODS
2.1. Study Design and Subjects
This was a hospital-based, retrospective observational study conducted from March to June 2021 at the Bethesda Hospital's Stroke Center, Yogyakarta, Indonesia. The study's inclusion criteria were as follows: (i) male or female above the age of 18 years old, (ii) stroke onset within 24 hours, (iii) complete radiological and laboratory data. Patients were excluded if they were: (i) discharged according to medical advice, (ii) referred to another hospital, (iii) patients with infection from any origin, (iv) received anti-inflammatory or immunosuppressant medications. During this study, all participants got the same treatment and underwent the same rehabilitation, adjusted to each patient's needs.
2.2. Clinical Data
The clinical data obtained included demographic information (age, gender), medical history (hypertension, diabetes mellitus, dyslipidemia, and atrial fibrillation), muscle strength, and laboratory findings. Venous blood samples of all subjects were taken upon admission and analyzed within 24 hours of admission. The NLR was calculated as the ratio of neutrophil to lymphocyte count.
2.3. Stroke Severity and Functional Outcome
The National Institutes of Health Stroke Scale (NIHSS) score was used to determine stroke severity on admission; meanwhile, the Barthel Index (BI) was used to determine functional outcomes 30 days following stroke onset. The NIHSS score measures stroke severity on a scale of 0 to 42, with higher scores indicating more severe stroke severity [10]. Stroke severities were classified into two categories based on the National Institutes of Health Stroke Scale (NIHSS) on admission: those with mild stroke (NIHSS <6) and those with moderate-to-severe stroke (NIHSS ≥6) [11]. The Barthel Index (BI) is a 10-item assessment that measures basic activities of daily living (ADL) on a scale ranging from 0 to 100 [12]. A previous study used a cutoff score of 50 for the Barthel Index to define a favorable outcome; a score of ≥50 indicates a favorable outcome, whereas a score of <50 indicates an unfavorable outcome [13].
2.4. Statistical Analysis
Percentages are used to describe categorical variables, while medians represent numerical variables (interquartile range). After adjusting for confounding variables, multivariate logistic regression was performed to determine whether higher leukocyte count and NLR predicted worse stroke severity and functional outcome in patients with acute ischemic stroke. Potential confounding variables included age, gender, hypertension, diabetes mellitus, dyslipidemia, atrial fibrillation, muscle strength, random blood glucose, total cholesterol, triglycerides, low-density lipoproteins, and high-density lipoproteins. Odds ratios were obtained with 95% confidence intervals. Statistical significance was defined as a 2-sided p-value of <0.05. The area under the curve (AUC) and 95% confidence intervals for the neutrophil-to-lymphocyte ratio and leukocyte count were determined using receiver operating characteristic (ROC) curve analysis. The analysis was conducted using SPSS Statistics version 21.0 software. Missing data will be imputed.
3. RESULTS
3.1. Baseline Characteristics
The current study included 122 subjects; 65 (58%) were male and 47 (42%) were female, with the majority of subjects aged 51 to 70. The baseline characteristics of the subjects are shown in Table 1. Hypertension (53.6%) was the most frequent comorbidity among all subjects, followed by dyslipidemia (26.8%), diabetes mellitus (24.1%), and atrial fibrillation (1.8%). The majority of subjects (64.3%) demonstrated muscle power on a scale of 4, which equates to active movement against gravity and resistance, according to the Medical Research Council's (MRC) muscle strength scale. The median leukocyte count and NLR obtained at admission were 9.10 x 109/L (4.89 - 18.40) and 2.86 (0.64 - 21.00) consecutively.
3.2. Stroke Severity and Functional Outcome
Thirty-six (32.1%) subjects had mild stroke severity and 76 (67.9%) subjects had moderate to severe stroke severity on admission, whereas 7 (6.3%) subjects had an unfavorable functional outcome and 105 (93.8%) subjects had a favorable functional outcome 3 months following stroke onset (Table 1).
| n= 112 | |
|---|---|
| Age (years) ̶ n(%) | |
| ≤ 50 | 10 (8.9%) |
| 51-60 | 38 (33.9%) |
| 61-70 | 38 (33.9%) |
| > 70 | 26 (23.2%) |
| Gender ̶ n(%) | |
| Male | 65 (58.0%) |
| Female | 47 (42.0%) |
| Comorbidities ̶ n(%) | |
| Hypertension | 60 (53.6%) |
| Diabetes mellitus | 30 (26.8%) |
| Dyslipidemia | 27 (24.1%) |
| Atrial fibrillation | 2 (1.8%) |
| Muscle strength (MRC scale) ̶ n(%) | |
| 0-no contraction | 0 (0%) |
| 1-Flicker or trace of contraction | 2 (1.8%) |
| 2-Active movement, with gravity eliminated | 8 (7.1%) |
| 3-Active movement against gravity | 25 (22.3%) |
| 4-Active movement against gravity and resistance | 72 (64.3%) |
| 5-Normal power | 5 (4.5%) |
| Stroke Severity | |
| Mild stroke severity (NIHSS <6) | 36 (32.1%) |
| Moderate to severe stroke severity (NIHSS ≥6) | 76 (67.9%) |
| Functional Outcome | |
| Unfavorable functional outcome (BI <50) | 7 (6.3%) |
| Favorable functional outcome (BI ≥50) | 105 (93.8%) |
| Laboratory Findings ̶ Median (IQR) | |
| Leukocyte count (x109/L) | 9.10 (4.89 - 18.40) |
| NLR | 2.86 (0.64 - 21.00) |
| Random blood glucose (mmol/L) | 8.02 (5.33 - 41.90) |
| Total cholesterol (mmol/L) | 10.96 (5.63 - 17.98) |
| Triglycerides (mmol/L) | 9.75 (3.00 - 35.46) |
| Low-density lipoproteins (mmol/L) | 7.42 (3.44 - 12.54) |
| High-density lipoproteins (mmol/L) | 2.25 (1.00 - 3.70) |
Higher leukocyte count (OR 1.357, 95% CI, 1.063-1.734, p: 0.014) and NLR (OR 1.609, 95% CI, 1.201-2.155, p: 0.001) were significantly related to a worse functional outcome 3 months after stroke onset. Higher leukocyte count (OR 1.247, 95% CI 1.056-1.473, p 0.009) was also associated with an increased risk of worse stroke severity on admission. Meanwhile, a higher NLR (OR 1.039, 95% CI, 0.895-1.206, p = 0.167) was not significantly related to an increased risk of worse stroke severity on admission. Following adjustment for age, gender, hypertension, diabetes mellitus, dyslipidemia, atrial fibrillation, muscle strength, random blood glucose, total cholesterol, triglycerides, low-density lipoproteins, and high-density lipoproteins, higher leukocyte count was found to be an independent risk factor for moderate to severe stroke (OR 1.391, 95% CI, 1.121-1.725, p: 0.003) and unfavorable functional outcome (OR 1.434, 95% CI, 1.068-1.925, p: 0.017) (Table 2).
| - | Leukocyte Count on Admission | NLR on Admission |
|---|---|---|
| Moderate to severe stroke severity (NIHSS ≥6) | ||
| Crude OR (95% CI) | 1.247 (1.056-1.473; p: 0.009) | 1.039 (0.895-1.206; p: 0.167) |
| Adjusted OR (95% CI) | 1.391 (1.121-1.725; p: 0.003) | 1.181 (0.947-1.474; p: 0.140) |
| Unfavorable functional outcome (BI <50) | ||
| Crude OR (95% CI) | 1.357 (1.063-1.734; p: 0.014) | 1.609 (1.201-2.155; p: 0.001) |
| Adjusted OR (95% CI) | 1.434 (1.068-1.925; p: 0.017) | 1.246 (0.905-1.716; p: 0.177) |
In the receiver operating characteristic curve analysis, The optimal cutoff value of leukocyte count for prediction of stroke severity was 8.94 x 109/L with a sensitivity of 57.9% and a specificity of 58.3% (area under the curve: 0.651, 95% CI 0.545-0.757; p: 0.010), and for prediction of unfavorable functional outcome was 9.44 x 109/L with a sensitivity of 57.1% and a specificity of 62.9% (area under the curve: 0.752, 95% CI 0.595-0.910 p: 0.026) (Fig. 1). The optimal cutoff value of NLR for predicting stroke severity was 2.81 with a sensitivity of 53.9% and a specificity of 52.8% (area under the curve: 0.517, 95% CI 0.401-0.633; p: 0.772), and for prediction of unfavorable functional outcome was 3.85 with a sensitivity of 71.4% and a specificity of 71.4% (area under the curve: 0.883, 95% CI 0.773-0.993; p: 0.001) (Fig. 2).
The results (OR) are reported per 1 increase in NLR and per 1x109 increase in leukocyte count.
Multivariable model: adjusted for age, gender, hypertension, diabetes mellitus, dyslipidemia, atrial fibrillation, muscle strength, random blood glucose, total cholesterol, triglycerides, low-density lipoproteins, and high-density lipoproteins.
4. DISCUSSION
This clinical study aimed to evaluate whether the neutrophil-to-lymphocyte ratio and leukocyte count are associated with stroke severity and functional outcome in acute ischemic stroke patients. Our findings indicate that a higher NLR is associated with worse stroke severity and a less favorable 3-month functional outcome, whereas a higher leukocyte count is significantly associated with worse stroke severity and a worse 3-month functional outcome. Numerous studies have established that leukocyte count is a significant independent predictor of stroke severity, disability, and overall mortality following ischemic stroke [14, 15], Furthermore, an elevated leukocyte count has been linked to recurrent ischemic stroke and the presence of large vessel occlusion [16, 17].
Immune cell activation is one of the earliest biological reactions discovered following ischemic stroke, indicating that the ischemic brain and the peripheral immune system are closely related. Ischemic brain injury causes a local inflammatory response and a systemic and sterile inflammatory response [18]. A prior study indicated that shortly following the onset of ischemic stroke, the neutrophil count increased exponentially, whereas the lymphocyte count decreased exponentially [19].
At stroke onset, the hypothalamo-pituitary-adrenal axis, the sympathetic–adrenal–medullary axis, and the parasympathetic nervous system (vagus nerve) are all implicated in immune response modulation [20]. During the first 24 hours following stroke onset, growth factors and chemokines promote neutrophils in the bone marrow, facilitating neutrophil transport and contributing to neutrophilia [21, 22]. Elevated catecholamine levels are related to increased lymphocyte apoptosis, leading to a reduction in peripheral blood lymphocytes [23-25]. Lymphocytes aggregate in the ischemic brain three to six days following stroke, whereas neutrophils accumulate sooner [26].
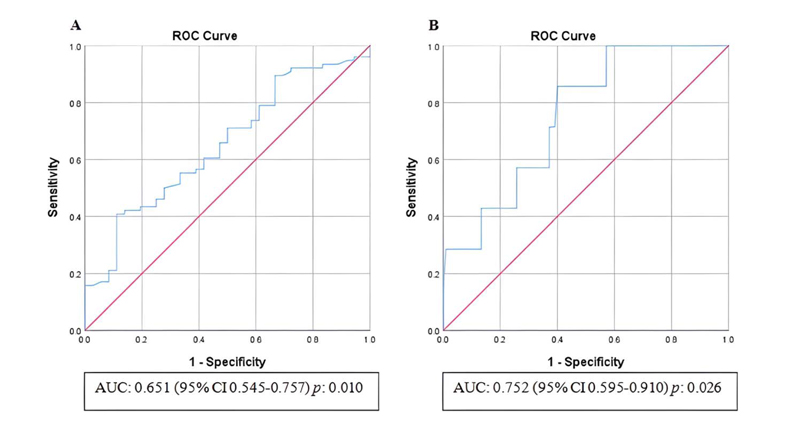
Abbreviations: AUC, Area under the curve; CI, Confidence interval.

Abbreviations: AUC, Area under the curve; CI, Confidence interval.
The inflammatory process is critical in ischemic stroke, and a higher leukocyte count in individuals with acute ischemic stroke is correlated with a worse outcome. The effect of leukocyte recruitment is controversial; while these cells may contribute to tissue regeneration, their deleterious consequences are more pronounced [27]. Increased numbers and activity of circulating leukocytes have been previously hypothesized to actively contribute to organ ischemia by increased adhesion to and disruption of the endothelium [28]. Proteases, inflammatory mediators, and free radicals released by leukocytes may cause proteolytic and oxidative damage to endothelial cells [16]. Moreover, since leukocytes are larger than all other blood cells, they may directly clog the microvasculature [29]. Some studies hypothesized that the negative effect of the increased neutrophil count could be related to neutrophils releasing matrix metalloproteinase-9, free oxygen radicals, and other inflammatory mediators, resulting in disruption of the blood-brain barrier (BBB), brain edema, and subsequent brain injury [4-7]. The lymphocytes' role in acute ischemic stroke is debatable. Several studies established that lymphocytes can repair inflammation-induced damage [4, 30]. However, other studies have reported that lymphocytes can emit proinflammatory cytokines and cytotoxic chemicals, resulting in brain injury in ischemia patients [30, 31]. Leukocyte count has also been demonstrated to be an independent predictor of recurrent coronary events in previous studies [32, 33]. Moreover, recent publications have also highlighted the association between NLR and carotid artery stenosis [34, 35].
Several limitations should be considered. Laboratory results for this study were only available at the time of admission. Since stroke-induced inflammation is a dynamic process that could vary dramatically even within the first 24 hours following a stroke, further research is needed to examine blood samples at various time intervals following a stroke. This study made no distinction between subtypes of ischemic stroke. In future investigations, the NLR and leukocyte count should be evaluated in various subtypes of ischemic stroke. Patients who underwent reperfusion therapy and those who did not get reperfusion therapy were not divided in this study. In addition, this study did not further differentiate the types of anti-diabetic medicines the patients received. Future studies are necessary to categorize patients on reperfusion therapy since reperfusion might cause an improvement in the inflammatory process, as well as identify patients' anti-diabetic medicines since some medications may have anti-inflammatory activity.
CONCLUSION
Our study indicates that leukocyte count is an independent predictor of stroke severity upon admission and unfavorable functional outcome. Additionally, the findings of this study provide possibilities for future studies into the potential utility of anti-inflammatory therapy for patients with acute ischemic stroke.
AUTHORS' CONTRIBUTIONS
RTP is responsible for the investigation, initial manuscript, data processing, and supervision. VV is responsible for the initial manuscript, data processing, and editing.
LIST OF ABBREVIATIONS
| NIHSS | = The National Institutes of Health Stroke Scale |
| BI | = Barthel Index |
| AUC | = Area Under the Curve |
| ROC | = Receiver Operating Characteristic |
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
This study was approved by the Ethics Committee of Bethesda Hospital, Yogyakarta, Indonesia, with an approval number of 19/KEP-RSB/II/21.
HUMAN AND ANIMAL RIGHTS
No animals were used for studies that are the basis of this research. All the humans were used in accordance with the ethical standards of the committee responsible for human experimentation (institutional and national), and with the Helsinki Declaration of 1964 and its subsequent amendments.
CONSENT FOR PUBLICATION
The authors acquired the patients' written consent for the involvement in and publishing of this study.
STANDARDS OF REPORTING
STROBE guidelines were followed.
AVAILABILITY OF DATA AND MATERIALS
The data that support the findings of this study are available from the corresponding author, [R.T.P], on special request.
FUNDINGS
This study was fully funded by two authors.
CONFLICT OF INTEREST
The authors declare no conflict of interest, financial or otherwise.
ACKNOWLEDGEMENTS
We would like to express our sincere gratitude to the participants for their participation in this study. The abstract of this study has been presented in ASEAN Neurological Association 2021, 17th World Congress on Controversies in Neurology (CONy), and the 6th Edition of the International Conference on Neurology and Brain Disorders.


