REVIEW ARTICLE
Insomnia – Is it a Symptom or a Disorder?
Gulcin Benbir Senel1, *
Article Information
Identifiers and Pagination:
Year: 2022Volume: 16
E-location ID: e1874205X2208150
Publisher ID: e1874205X2208150
DOI: 10.2174/1874205X-v16-e2208150
Article History:
Received Date: 7/2/2022Revision Received Date: 1/4/2022
Acceptance Date: 23/5/2022
Electronic publication date: 07/10/2022
Collection year: 2022

open-access license: This is an open access article distributed under the terms of the Creative Commons Attribution 4.0 International Public License (CC-BY 4.0), a copy of which is available at: https://creativecommons.org/licenses/by/4.0/legalcode. This license permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Abstract
Insomnia disorder is a common public health problem with a prevalence of approximately 2-5% of the population. It is of major importance to differentiate the insomnia disorder from the isolated symptoms and normal variants, and to define the secondary or associated conditions. Insomnia was mainly classified as acute and chronic insomnia disorder based on the 3rd edition of the International Classification of Sleep Disorders. Many models have been developed to explain the underlying mechanisms of insomnia, such as the Drosophila model, the cognitive model, the psychobiological inhibition model, the neurocognitive model, stimulus control model, hyperarousal model and the “3P model” (Spielman model). Optimizing the environmental conditions, lifestyle changes and elaborating the triggering factors are the first step in the management of insomnia disorders.
1. INTRODUCTION
Insomnia is a significant public health problem commonly encountered in the general population as a symptom, and holds up to about 50-60% of life-long prevalence [1]. On the other side, insomnia disorder is reported only in approximately 2-5% of the population. The clinicians should therefore pay attention when to diagnose insomnia disorder in an individual complaining of insomnia. The isolated symptoms and normal variants, such as being a “short sleeper” or having “excessive time in bed” should be carefully excluded in these individuals with a careful and detailed anamnesis of sleep habits. Secondary or associated conditions with insomnia should also be clarified and excluded.
1.1. Description and Diagnosis
The diagnosis of insomnia disorder, as one of the sleep disorders, is made according to the current classification defined by the American Academy of Sleep Medicine [2]. The diagnostic criteria of insomnia disorder on the basis of the third edition of International Classification of Sleep Disorders are shown in Table 1.
The first diagnostic criterion is the presence of the complaint of insomnia. There may be a problem in initiating sleep, maintaining sleep, or early awakening than the desired
timing may be the main complaint. Either of these may define insomnia. In adolescents or young adults, showing resistance to going to bed or needing a pre-formed condition to sleep, such as the presence of a parent or a toy, may constitute the main complaint of insomnia. These features may be reported by the parents of a child or by the caregiver of a patient. When a physician faces a patient with either a single or a combination of these complaints, a detailed questioning about the sleep hygiene of the patient should be made to differentiate the symptom “insomnia” from the “insomnia disorder”. Also, other conditions mimicking insomnia, such as normal variants like “short-sleeper”, should be excluded. For these reasons, a detailed questioning should include the following questions:
- Is there a regular timing to go to bed and to get out of the bed? If not, the earliest and the latest hours should be noted.
- What is the sleep latency following going to bed? The earliest and the latest minutes of hours should be noted.
- What is the total duration spent within the bed? How many hours of this duration have approximately been spent sleeping?
- Is there a difference between working days and the weekends?
- Is there a difference during longer vacations?
- Are there daytime naps? How many times, and for how long?
| Criteria A-F must all be met for the diagnosis of chronic insomnia disorder: A. The patient (or parents or caregivers) reports at least one of the following: 1. Difficulty initiating sleep, 2. Difficulty maintaining sleep, 3. Waking up earlier than desired, 4. Resistance to going to bed on an appropriate schedule, or 5. Difficulty sleeping without parent or caregiver intervention. B. The patient (or parents or caregivers) reports at least one of the following symptoms related to the nighttime difficulty in sleeping: 1. Fatigue or malaise, 2. Decrease in attention, concentration, or memory, 3. Impairments in social, family, occupational or academic performance, 4. Mood disturbance or irritability, 5. Daytime sleepiness, 6. Behavioral problems (such as hyperactivity, impulsivity or aggression), 7. Decreased motivation, energy, or initiative, 8. Proneness for errors or accidents, 9. Concerns about or dissatisfaction with sleep. C. The complaints shall not be explained purely by the inadequate time allotted for sleep or inadequate environmental conditions for sleep. D. The symptoms occur at least three times per week. E. The symptoms are present for at least three months. F. The condition is not better explained by another sleep disorder. |
| Criteria A-C are the same in diagnosing acute insomnia disorder. Criterion D states that the symptoms are presently less than three months. Criterion E is the same as Criterion F. |
A comprehensive understanding of the regular sleep hygiene of the patients aids not only a correct diagnosis of the insomnia disorder but also helps to differentiate its sub-types, and thus planning a rational approach to its management. The second criterion is the need for a consequence of night-time symptoms for daily life living. A variety of daily living activities may be affected secondary to insomnia because of fatigue, tiredness, problems in attention, concentration and memory, and excessive daytime sleepiness, resulting in impairments in school, family, social or working performances, mood disturbances, or behavioral disturbances. The important point should be the cause and effect relationship between the night-time complaints and the daytime consequences. As the problems linked to insomnia may actually be caused by a different underlying cause, the clinician should pay specific attention to the delineation of a possible underlying cause of insomnia and night-time symptoms.
The third criterion emphasizes the need for the appropriate environmental conditions for a healthy sleep. If there is not enough time or space to sleep, either due to personal programming (such as work or social activities) or due to living conditions (such as living in a dormitory), the complaint of insomnia should not be diagnosed as a disorder. Being unable to sleep due to noise pollution, for example, or excessive light exposure, is accepted to be related to impaired sleep hygiene, but not to insomnia disorder. On these bases, the complaint of insomnia should be present in spite of appropriate timing and suitable environmental conditions for sleep to be diagnosed as insomnia disorder. From another stand view, insomnia may not be directly related to inappropriate environmental conditions. The physician should ask whether there is a particular behavior or a habitual task before bedtime, which may actually be triggering or worsening insomnia, such as reading a book in bed or having daytime naps.
Another important criterion in the diagnosis of insomnia disorder is the duration and frequency of insomnia. For the diagnosis of “chronic insomnia disorder”, the insomnia should last for at least three months with a frequency of at least three times in a week. If the complaint of insomnia is present for more than a month but less than three months, it is referred to as the “acute insomnia disorder”. In some cases, a patient might have insomnia lasting for many months or years, but the frequency may be less than three per week. In such a case, this patient should also be assigned as having chronic insomnia disorder, given the persistence of the complaints over a long time. The time at the onset of the symptoms should also be carefully questioned; insomnia starting from very early years of life may give a clue for the idiopathic subtype of chronic insomnia disorder. The presence of a precipitating event should also be noted, which may preoccupy the psychophysiological subtype of chronic insomnia disorder.
Last but not least, the complaint of insomnia, night-time symptoms and the daytime consequences should not be better explained by another sleep disorder. As stated above, comorbid conditions may lead to insomnia, and the diagnosis of insomnia disorder should be avoided only if they are the sole cause of insomnia. Otherwise, if another condition is associated with the insomnia disorder and the complaints is not the only cause, the diagnosis of the insomnia disorder is made. The use of some medications may also distort the condition, and the complaints may not fulfill the diagnostic criteria. Physicians should be careful about the drugs and/or substances that may interfere with insomnia and night-time problems without these medications/substances should be applied for diagnosis.
A patient admitted with a complaint of insomnia or a child/elderly/dementia patient brought to the clinic by their parents/caregivers deserves attention to obtain a detailed history of sleep habits before and after the onset of insomnia, the clinical features of insomnia, and past medical history. On the basis of the above-mentioned assessments, a schematic approach to a patient with insomnia is summarized in Fig. (1).
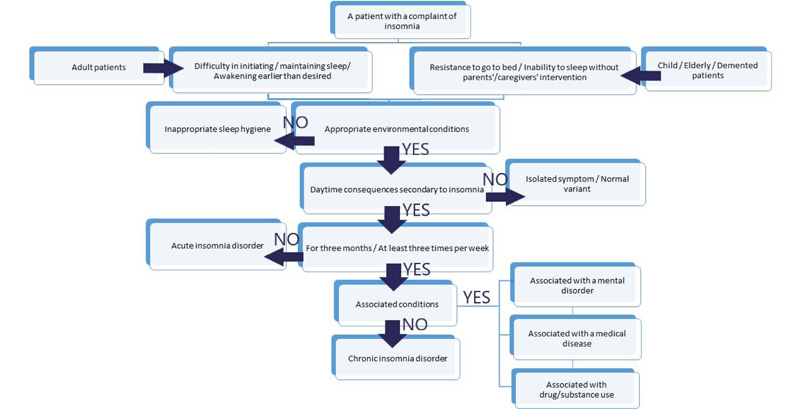 |
Fig. (1). The approach to a patient with insomnia. |
1.2. Classification
Insomnia was mainly classified as chronic and acute insomnia disorder in the latest and 3rd edition of the International Classification of Sleep Disorders [2]. Other insomnia disorders and isolated symptoms, and normal variants have also been defined (Fig. 2). In addition, the clinical and pathophysiological subtypes have been defined for chronic insomnia disorder. On this basis, psychophysiologic, idiopathic, paradoxical insomnia and insufficient sleep syndrome are described.
Psychophysiological insomnia is mainly characterized by increased arousal and mal-adaptive sleep-preventing behaviors, such as increasing the time spent in bed or doing some other activities than sleeping in bed, like reading a book, which results in or worsens insomnia. These patients have complaints of difficulty in initiating sleep at home, which resolves when they go to sleep in novel settings, or if they can stop trying to sleep. Idiopathic insomnia typically presents with a longstanding complaint of insomnia starting from the early years of life, probably due to genetic or congenital impairments either in sleep-inducing or wake-inducing systems, or both. It usually becomes stable over time and lasts lifelong. Paradoxical insomnia may also be called sleep-state misperception, characterized by a mismatch between subjective and objective measures of the quantity and the quality of sleep. Inadequate sleep hygiene results from routine daily-living activities, which are not compatible for the maintenance of good sleep hygiene. A highly variable sleep schedule, habits like daytime napping, using sleep-preventive substances, such as caffeine or tobacco, close to bedtime, and having emotionally upsetting or mental/physical demanding activities close to bedtime may trigger insomnia.
In children, chronic insomnia disorder is named as the behavioral insomnia of childhood, which has three subtypes, including sleep-onset association type, limit-setting type, and mixed type. Actually, in all subtypes, an improper sleep training by the parents or caretakers is presumed to be the underlying reason of insomnia disorder in the pediatric age group. In the sleep-onset association type, the child is dependent on specific environmental conditions, such as having an object or a setting to initiate sleep, or returning back to sleep after awakening in the middle of the night. In the limit-setting type, the child refuses to go to bed at bedtime.
Insomnia associated with another mental or medical disorder or with a drug or substance is regarded to result from these underlying etiologies, or to be secondary to the co-occurring conditions. The most common psychiatric conditions associated with insomnia are mood disorders, anxiety and personality disorders. Among other medical conditions, painful diseases, immobilization and breathing disturbances are most commonly encountered. Insomnia may also result from or be secondary to the use of or withdrawal from a drug or substance. The differential diagnosis of the underlying etiology based on the type of the insomnia complaints is summarized in Fig. (3).
 |
Fig (2). The clinical and pathophysiological subtypes of chronic insomnia disorder. |
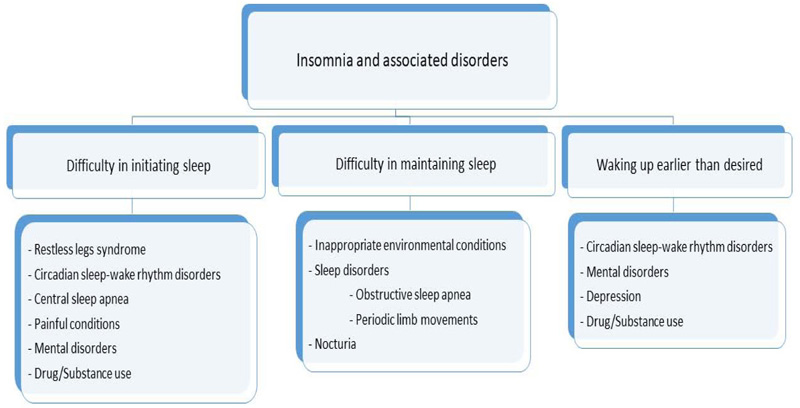 |
Fig. (3). The differential diagnosis of insomnia based on the type of complaints. |
Among isolated symptoms and normal variants are having excessive time in bed and being a short sleeper. Some individuals have long periods of wakefulness in bed during night time, but they do not have associated complaints of insomnia, such as daytime consequences. This condition is observed especially in retired individuals, who routinely have excessive time in bed habitually. Individuals sleeping less than six hours per night without other accompanying symptomatology are defined as short sleepers. The clinical significance of the isolated symptoms and normal variants remains to be answered. Although metabolic or cardiovascular consequences have been linked to the short sleep duration in some studies, these results have been handicapped due to the possibility of associated problems causing short sleep duration. At present, short sleepers are assigned as normal variants as long as they do not fulfill the diagnostic criteria given above.
1.3. Epidemiology
The prevalence of acute insomnia disorder is around 15-20% per year in adults. The prevalence of chronic insomnia disorder is about 10% of the general population, which increases with age [3, 4]. Behavioral insomnia of childhood may show differences in identification depending on the cultural definitions, though an overall prevalence of 3-10% is reported in puberty and adolescents. The prevalence of insomnia disorder increases in monozygotic twins and in first-degree relatives, though a familial pattern has not been documented [5]. Both acute and chronic insomnia are known to be more prevalent in women and in older age groups. Insomnia is associated with lower income level, time spent watching TV, tea consumption in the evening and smoking. Its prevalence also increases in the presence of medical diseases, such as hypertension, diabetes and heart diseases [3].
1.4. Pathophysiology
Many models have been developed to explain the underlying mechanisms of insomnia. As sleep-wake habits, such as the duration and timing of sleep, are regulated by circadian and other genes, many researchers have suggested genetic mechanisms in the etiology of insomnia [6]. It was first demonstrated in Drosophila flies that the genes associated with cell surface signaling and neuronal activity, such as PER or Clock genes, may have implications for the pathophysiology of insomnia. Drosophila model, an analogue of human insomnia, suggests that insomnia develops depending on the predisposing factors characterized by strong genetic susceptibility [7]. The current evidence also supports that the heritability with the involvement of multiple genes plays a role in the development of insomnia disorder.
The cognitive model of insomnia defines that individuals, who are genetically susceptible to anxiety and unpleasant thoughts, develop insomnia [8]. These individuals have excessive anxiety because of not getting enough sleep or because of the daytime consequences of night-time disturbances, which result in triggering or worsening of insomnia. Changes in the microstructural elements of sleep (such as sleep spindles) have been shown in paradoxical insomnia, correlated with the increased level of uncontrollable and persistent anxiety in the Penn State Worry Questionnaire and the extroversion dimension in the Eysenck Personality Questionnaire [9, 10]. When the focus and selective attention to initiate sleep increases, the awareness of the environment also increases, leading to the hyperarousal state. Psychobiological inhibition model states that although stress triggers both psychologic and physiologic arousal to cause acute insomnia disorder, chronic insomnia disorder is less associated with hyperarousal but more associated with the inability to inhibit the wakefulness [11].
The neurocognitive model suggests the attenuation of the physiologic mesograde amnesia of sleep, and concentrates on the role of the maladaptive behavioral strategies in the development of the chronic insomnia disorder, and suggests that the repeated association of the sleep-related cues with the wakefulness creates conditioned arousal [12, 13]. Stimulus control model explains the association of insomnia with these maladaptive behaviors; when the environmental conditions, such as the bed or other bedtime activities, are paired not only with sleep but other behaviors, the insomnia disorder develops due to the loss of sleep-specific cues [14-16]. For example, if a patient spends excessive time in bed to cope with insomnia complaints, and is occupied with some activities, such as reading, other than sleep in the bed, stimulus dyscontrol will result in the development of the insomnia disorder.
A hyperarousal state characterized by both physiologic cortical hyperarousal and peripheral autonomic activation (including somatic, cognitive, and cortical components) is believed to occur at bedtime, resulting in insomnia [13, 17]. The initial data arose from animal experiments; “Cage change model” was based on the change of the cages of the rats, which triggered stress and activated arousal-related neuronal circuits [18, 19]. Many studies in humans have shown changes in cortisol levels, metabolic rate, body temperature, skin resistance, heart rate variability, or electroencephalographic spectral analysis, supporting the presence of a hyperarousal state in insomnia. Studies have shown a decrease in delta and an increase in alpha activity in the spectral analysis during non-rapid eye movement (non-REM, NREM) sleep. It was suggested that this hyperarousal state is associated with an increased sensory processing and increased discrepancy between the subjective and objective time spent in sleep and wakefulness. The instability in REM sleep, on the other hand, has been hypothesized to be more closely related to the subjective complaints of insomnia [20]. The fragmentations from REM sleep with micro-arousals and awakenings were proposed to result in a higher perception of the time spent in wakefulness, contributing to the mismatch in the subjective and objective measures of sleep.
The “3P model” (Spielman model), the most widely accepted model of insomnia, incorporates these genetic, cognitive and hyperarousal mechanisms with the behavioral factors in a set of “predisposing”, “precipitating” and “perpetuating” factors (Fig. 4) [21, 22]. The predisposing factors define the genetic susceptibility of an individual to develop insomnia. The precipitating factors define a trigger or a stressor that initiates insomnia. These first two factors are especially associated with the development of insomnia. The perpetuating factors are the maladaptive behaviors that contribute to the maintenance of insomnia. Spending an increased time in other activities than sleep in bed is one of these behaviors. Having excessive time in bed perpetuates insomnia by leading to increased wakefulness and awareness of environmental stimuli. Activities, such as reading or listening to music in bed, perpetuate insomnia by creating conditioning to a stimulus.
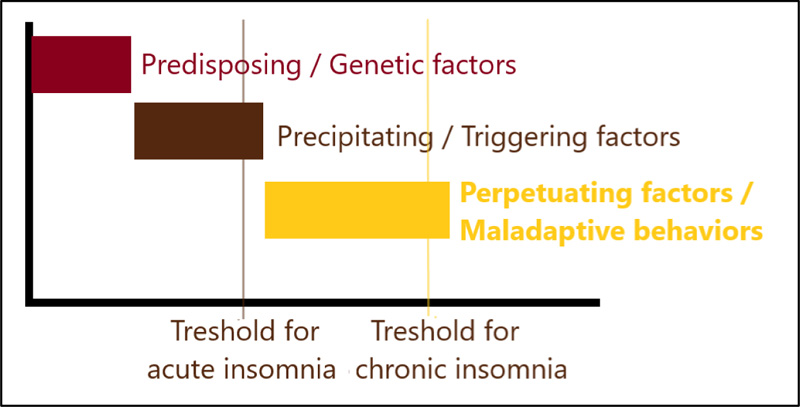 |
Fig. (4). The 3P model. |
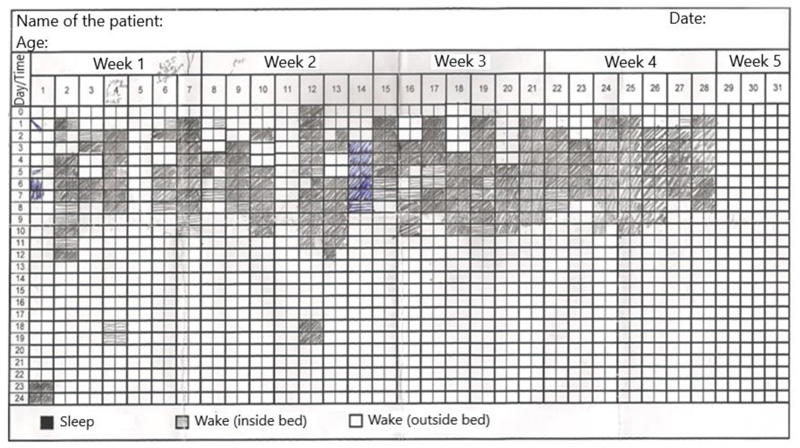 |
Fig. (5). An example of a sleep log. |
1.5. Scales Used in the Evaluation of Insomnia
1.5.1. Subjective Assessment
Besides clinical anamnesis, there are many scales developed in the evaluation of insomnia, probably because the diagnosis is mainly based on clinical data. Although objective measures, such as actigraphy and polysomnography (PSG), may give additional beneficial data related to patients with insomnia, also needed in the diagnosis of paradoxical insomnia, the detailed questioning of the subjective symptoms constitutes the mainstay in the evaluation of insomnia.
Sleep log, showing the schematic drawings of the sleep-wake patterns, demonstrates 14-day habitual sleep hygiene. So that not only the duration of sleep but the timing of the sleep, possible shifts in the circadian rhythm, daytime naps, and changes in insomnia from day to day are recorded and visualized. An example of a sleep log filled in by a patient is given in Fig. (5). This will help in understanding the maladaptive behaviors and guide the management of the patients.
In a study by Lomeli et al . [23], the scales that evaluate sleep characteristics are well-summarized in the pediatric population, adolescents and in adults. Brief infant sleep questionnaire, Pediatric sleep questionnaire, School sleep habits survey, Sleep survey for adolescents, Pittsburg sleep quality index, Pittsburgh insomnia rating scale, Insomnia symptom questionnaire, Athens insomnia scale, and Subjective assessment scale of sleep and dreams are only a few examples of these scales.
1.5.2. Objective Assessment
Actigraphy is a method that involves a device to be worn on the wrist or ankle, which records the limb movement activities and analyses the occurrence of the movements in order to estimate wakefulness and sleep based on mathematical algorithms. It provides objective and reliable data for some sleep parameters, consistent with PSG measures [24]. In patients with a suspicion or diagnosis of insomnia, the use of actigraphy was demonstrated to be valid against polysomnography [25, 26]. For the diagnosis of paradoxical insomnia, a subtype of chronic insomnia disorder, the actigraphy or PSG should be performed as an objective measure of sleep in order to delineate the discrepancy between the subjective complaints and the objective measures. Actigraphy involves a reliable, cheap, and easily accessible device, permitting long-term recordings obtained at home, with additional parameters to be used in need.
Polysomnography is not indicated in the diagnosis of acute or chronic insomnia disorder. However, it may be used as one of the objective sleep measurements for the diagnosis of paradoxical insomnia. In addition, in patients with chronic insomnia disorder characterized by the difficulty in the maintenance of nighttime sleep associated with frequent arousals, PSG may help in the identification of other possible sleep disorders, such as sleep apnea or periodic limb movement disorders that might be responsible for insomnia. Also, in patients with insomnia who fail to show a good response to the cognitive behavioral therapy and/or pharmacological treatments, PSG may help in the detection of any other underlying pathology that may not be evident in the clinical anamnesis. If performed, PSG may show an increase in the sleep latency (SL >30 minutes), an increase in the wakefulness after sleep onset (WASO >30 minutes), a decrease in the total duration of sleep (total sleep time, TST <6 hours), an increase in the superficial sleep stages (NREM 1 and 2 stage), and a decrease in the slow delta sleep (NREM 3 stage) [2, 26]. The night-to-night change tends to be more pronounced in patients with insomnia compared to those having a good sleep. Although most patients underestimate their time spent in sleep, as shown by PSG, it is very pronounced in paradoxical insomnia; the discrepancy is more than two hours, or the actual total sleep time is twice as estimated.
1.6. Management of Insomnia Disorder
Optimizing environmental conditions, inducing lifestyle changes and elaborating the triggering factors should be done in all insomnia disorders. Sleep hygiene, stimulus control, cognitive behavioral therapy and relaxation methods should also be planned in all subtypes of chronic insomnia disorder [27]. In addition, cognitive remodeling and awareness-based remodeling techniques may be beneficial, especially in idiopathic and paradoxical types of insomnia. Sleep hygiene teaches the correct behaviors before bedtime and prepares the body for sleep. Sleep restriction increases the homeostatic drive for sleep and regulates the circadian rhythm. Stimulus control is important for establishing the interaction between sleep and environmental conditions. Relaxation methods aim to clarify, decrease or eliminate the factors causing physical and/or cognitive arousal at bedtime. Cognitive remodeling aims to change the misbeliefs related to sleep and insomnia, and remodels the inappropriate thoughts that cause insomnia to become chronic. The awareness-based remodeling techniques (breathing, physical sensations, exercises of thoughts and feelings) are usually used together with behavioral cognitive therapy. They aim to decrease the biological and physiologic hyperarousal state, as well as the ruminations related to daytime pursuits, by directing the attention of the patients to sleep.
| Drugs | Effect of Action | Indications | Dosage | Tmax / T1/2 | FDA Approval | Side Effects |
|---|---|---|---|---|---|---|
| Benzodiazepine Group of Hypnotics | ||||||
| Triazolam | Selective GABA-A agonists | Difficulty in initiating sleep | 0.125-0.250 mg | 1-2 hr / 2-6 hr | + | Sedation Confusion Anterograde amnesia Rebound insomnia |
| Temazepam | Difficulty in initiating and maintaining sleep | 7.5-30 mg | 1-2 hr / 8-15 hr | + | ||
| Non-Benzodiazepine receptor agonists | ||||||
| Zopiclon | GABA-A agonists | Difficulty in initiating > maintaining sleep | 3.75-7.5 mg | 1.5-2 hr / 5 hr | - | Dizziness Sleepiness GI effects |
| Eszopiclon | 1-3 mg | 1-1.5 hr / 6 hr | ||||
| Zolpidem | 1.75-10 mg | 1-2 hr / 2-6 hr | ||||
| Zaleplon | Difficulty in initiating sleep | 5-20 mg | 1 hr / 1.5-2 hr | + | ||
| Melatonin and agonists | ||||||
| Melatonin | On supra-chiasmatic nucleus | Difficulty in initiating > maintaining sleep | 3-12 mg | 1 hr / 1 hr | + | Dizziness GI effects Sedation Headache Anxiety Hyperhidrosis |
| Ramelteon | M1 and M2 agonists | 8 mg | 0.75-1 hr / 1-1.5 hr | |||
| Sirkadin | 2 mg | 0.75-3 hr / 3.5-4 hr | ||||
| Agomelatin | M and 5HT2c agonist | 25-50 mg | 2-3.5 hr / 12 hr | - | ||
| Orexin antagonists | ||||||
| Suvorexant | Orexin R1 and R2 antagonist | Difficulty in maintaining > initiating sleep | 5-20 mg | 2-3.5 hr / 12 hr | - | Headache Dizziness GI effects Sedation |
| Antidepressants | ||||||
| Doxepin | H1 antagonist | Difficulty in maintaining sleep | 2-6 mg | 2-8 hr / 20 hr | - | Weight loss Hyperhydrosis Priapism Cardiac rhythm disorder (Amitriptyline) Weight gain, hyperlipidemia (Mirtazapine) |
| Amitriptyline | H1, α1 and M antagonist | 10-75 mg | 2-8 hr / 30 hr | |||
| Trazodone | 5HT2A and α1 antagonist | 50-100 mg | 1-2 hr / 9 hr | |||
| Mirtazapine | H1 and 5HT2A/2C antagonist | 15-45 mg | 1-3 hr / 25 hr | + | ||
| Antipsychotics | ||||||
| Quetiapine | H1, α1, M1, 5HT and D2 antagonist | Difficulty in maintaining sleep | 25-50 mg | 1-2 hr / 6 hr | - | Dizziness Weight gain GI effects |
| Olanzapine | 2.5-5 mg | 4-6 hr / 20-54 hr | ||||
| Histamine antagonists | ||||||
| Diphenhydramine | H1 antagonist | Difficulty in maintaining sleep | 12.5-50 mg | 2-3 hr / 10 hr | - | Dizziness Sedation |
| Alpha-2-delta ligands | ||||||
| Gabapentin | Inhibit Ca flow and increase excitatory neuro-transmitters | Difficulty in initiating and maintaining sleep | 900-3600 mg | 3-4 hr / 6 hr | - | Dizziness Weight gain GI effects Mouth dryness Depression Thoughts of suicide |
| Pregabalin | 75-300 mg | 1 hr / 6 hr | ||||
CONCLUSION
Short and fast-acting drugs, such as benzodiazepines and Z-drugs, may be used for a short time in the treatment of acute insomnia disorder. In chronic insomnia disorder, the drug choice should be made on the basis of the type of the complaint, such as fast-acting drugs should be used in the presence of difficulty in initiating sleep and long-acting drugs should be preferred in the presence of difficulty in maintaining the sleep. Because the duration of the pharmacological therapy will be for months and even for years in some cases, benzodiazepines and Z-drugs should be avoided. The disorders associated with insomnia should also be taken into account in deciding the drug [28]. The antidepressant drugs may be chosen in the presence of mental disorders, or alpha-2-delta ligands may be helpful in the presence of movement disorders or painful conditions associated with insomnia. Although antipsychotic medications are not recommended in the treatment of insomnia disorder, they may be used in low doses in paradoxical insomnia, as it is accepted as a sleep misperception state. Combination therapy may also be used in some cases when necessary.
Behavioral insomnia of childhood is usually treated by non-pharmacologic approaches. The education of the parents and/or caregivers constitutes the mainstay of the treatment [29, 30].
Although a brief introduction to the treatment of insomnia is given here, it is beyond the scope of this article. The drugs prescribed for insomnia in adults are summarized in Table 2 [31, 32].
CONSENT FOR PUBLICATION
Not applicable.
FUNDING
None.
CONFLICT OF INTEREST
The author declares no conflict of interest, financial or otherwise.
ACKNOWLEDGEMENTS
Declared none.








