All published articles of this journal are available on ScienceDirect.
Simultaneous Onset of Alzheimer’s Disease in a Husband and Wife in Their Mid-Fifties: What Do We Really Know About Environmental Factors?
Abstract
Introduction:
Environmental factors can play a role in the pathogenesis of Alzheimer’s disease. We present a case of the simultaneous onset of Alzheimer’s disease in two middle aged adults.
Case presentation:
A married couple ages 54 year and 51-year-old female cohabiting together were diagnosed with Alzheimer’s disease within the same year. The patient’s both developed cognitive decline shortly after a major renovation of their property and followed a similar disease course. The diagnosis was supported by clinical presentation and tissue pathology on autopsy.
Conclusion:
Environmental factors may play a role in the pathogenesis of Alzheimer’s disease. Further understanding of the disease cascade is required.
INTRODUCTION
Whereas the genetic factors influencing the development and expression of Alzheimer’s disease pathology are well characterized, environmental factors are currently thought to play a marginal role. Factors such as prior closed head injury, post-menopausal estrogen deficiency, aluminum exposure, smoking, diabetes, atherosclerotic cardiovascular disease, and diet, among others, have been shown to confer only a modest increased risk, if any, and are only tangentially considered in the major pathogenic cascades that are presently hypothesized [1,2]. We present the simul-taneous onset of Alzheimer’s disease in a husband and wife, with both subjects experiencing cognitive dysfunction within the same month. The subjects had an extremely similar presentation and course of disease. Both were in their mid-fifties at the time of presentation showed progressive neurological decline with prominent memory loss, ex-perienced myoclonus late in their disease course (prompting referral to the National Prion Disease Pathology Surveillance Center), and expired 12 years after onset, within months of each other. Review of the family pedigree revealed no significant family history of dementia or other neurologic illnesses in multiple first degree relatives. The only historical finding of note was that both subjects had moved out of their home briefly while it was being remodeled, and both became symptomatic shortly after moving back in. At autopsy, the subjects had classic advanced Alzheimer’s disease, with Braak stage IV pathology which was otherwise identical inquantity and distribution of amyloid-beta, cerebral amyloid angiopathy, and neurofibrillary degeneration. While no specific toxin or other environmental cause was identified, these two cases raise the issue of epigenetic factors in Alzheimer’s disease that may be more robust than current literature indicates.
CASE HISTORY
Patients R.V. and P.V. were a married couple aged 54 and 51 respectively at time of presentation. R.V. presented with new onset cognitive dysfunction i and P.V. presented with new onset cognitive dysfunction and loss of memory and apraxia, both in 1995. In 1994, both husband and wife moved out of their home for a major renovation project that lasted approximately six months. During excavation, it was discovered that the property was used for dumping over one hundred years prior. The nature of the waste is unknown, but the couple began their cognitive decline within six months of moving back in to the residence.
R.V.’s symptoms began with cortical blindness, apraxia, and acalculia. His disease developed slowly over the following nine years with uncoordinated gait, photophobia, and myoclonus. In 2004, he began to have progressive hallucination and aggressive behavior. In his last six months of life, he became withdrawn, uncommunicative and extremely somnolent. P.V.’s disease abruptly declined over the course of the initial three years with the development of hallucinations, bizarre posture and eventually becoming uncommunicative with loss of facial expressions. Her disease progressed over nine more years with wasting despite increased caloric intake in her last months of life. He passed in 2008. She passed in 2009.
The husband (R.V.) was born in 1941 with no family history of dementia and no relevant past medical history. His wife (P.V.) was born in 1944 and had nine other siblings, one of which had elderly dementia with an onset at age 80, but no other family history of neurologic disorder. She had a past medical history of depression, smoking and alcoholism.
Family history of patient’s P.V. and R.V. showing no significant history of dementia.
Wife: P.V. Husband: R.V.
MATERIALS AND METHODOLOGY
Brain tissue was collected from each of the individuals. The sampled brain regions included: frontal cortex, occipital cortex and hippocampus. The tissue was fixed in routine formalin, embedded in paraffin and sectioned at 6 mm. Routine H&E staining was done on all three regions. Immunohistochemical staining was done on the frontal cortex and the hippocampus for tau and amyloid β proteins. The tissue was also examined for gross anatomical changes. Family history is reviewed. History of cognitive decline was obtained from family members.
RESULTS
At autopsy, the subjects had pathology which was otherwise identical in quantity and distribution of amyloid-beta, cerebral amyloid angiopathy, and neurofibrillary degeneration (Figs 2-4).
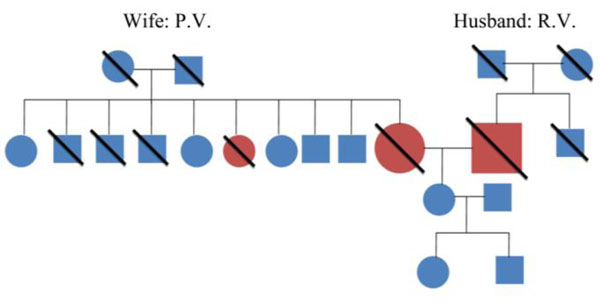
Family history of patient’s P.V. and R.V. showing no significant history of dementia.
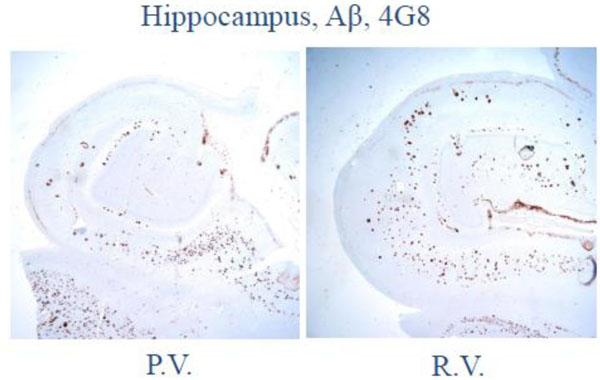
Low magnification photomicrograph on the hippocampus and adjacent temporal neocortex shows numerous senile plaques by amyloid-β (4G8) immunohistochemistry.

Low magnification photomicrograph of hippocampus and adjacent temporal neocortex shows extensive neurofibrillary degeneration on phosphor-tau (AT8) immunohistochemistry.
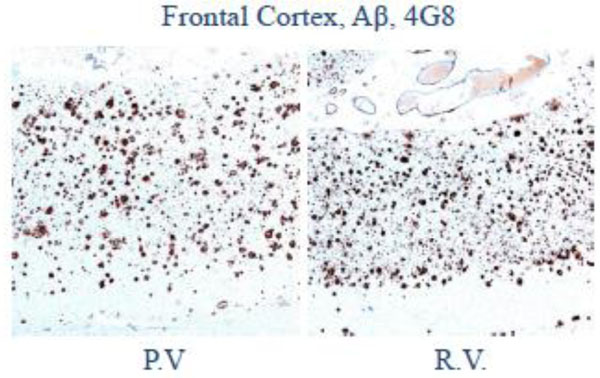
Low magnification photomicrograph of frontal cortex shows abundant senile plaques on amyloid-β (4G8) immunohistochemistry.
The couple was diagnosed with Alzheimer’s Disease according to the Consortium to Establish a Registry for Alzheimer’s Disease (CERAD) which uses standardized diagnostic criteria. Based on near identical neurofibrillary tangles of tau protein, they were also both given a Braak stage IV (advanced) diagnosis. At this stage, the tangles cause severe memory and cognitive impairment and the majority of patients will also suffer from dementia.
No specific toxin or other environmental cause was discerned.
DISCUSSION
There are several risk factors for Alzheimer’s Disease: advanced age, genetics, socioeconomic factors, and traumatic brain injury among others [3]. Although these factors have been well documented in the literature, cases such as this question the role that environmental factors may play in the degenerative decline towards Alzheimer’s dementia. Life-style related factors have been studied, showing positive correlations between them and Alzheimer’s disease. Diabetes mellitus type II, increased caloric intake, lack of exercise, smoking, and lack of intellectual activities have all shown to increase the risk for developing Alzheimer’s [4]. The causative role of these agents has been explained by their association with increased oxidative stress. Oxidative stress is caused by an accumulation of free radicals, eventually leading to neurologic damage [5].
It is understood through Fenton chemistry that reactive oxygen species are produced via interactions with transition metals (Al, Fe, Cu). Much like the life style factors discussed above, these transition metals could cause neurologic damage through oxidative stress [6]. This presents the possibility that the husband and wife’s Alzheimer’s disease could be explained by exposure to some toxin during or after their home renovation. Several studies have already reported the potential of transition metals as causative agents [7]. A study on rabbits by Sparks et al showed that the addition of low levels of copper (0.12 ppm) increased formation of amyloid plaques [8]. Aluminum accumulations have also been found in neurofibrillary tangles and are thought to promote aggregation of β-amyloid. Another study found that the risk of developing AD was higher in areas where the concentration of Al in drinking water was higher, though most of these conclusions are highly controversial [9].
Chemicals known to cause increased oxidative stress through various pathways are used in building materials, consumer products, etc. Is it mere coincidence that this couple developed such similar pathology in the same time frame? Were they both genetically predisposed from the start? Because no specific toxin was identified, we may never know, but the role of environmental factors in the pathogenesis of Alzheimer’s disease may be more prominent than previously thought.
CONCLUSION
The case above is a presentation of simultaneous onset of Alzheimer’s disease in a husband and wife, both of whom experienced cognitive dysfunction within the same month. As was noted the only finding of note, both historically and in their pedigree, was that both subjects had moved out of their home briefly while it was being remodeled, and both became symptomatic shortly after moving back in. Their clinical presentation was typical of Alzheimer’s disease and at autopsy both subjects were confirmed to have had classic advanced Alzheimer’s disease. While no specific toxin or other environmental cause was identified, this cases raises the question that there may be an important epigenetic factors in Alzheimer’s disease which is not currently suggested by the literature.


