All published articles of this journal are available on ScienceDirect.
Computed Tomographic Angiography as an Adjunct to Digital Subtraction Angiography for the Pre-Operative Assessment of Cerebral Aneurysms
Abstract
Objectives:
Computerized tomographic angiography (CTA) has emerged as a valuable diagnostic tool for the management of patients with cerebrovascular disease. The use of CTA in lieu of, or as an adjunct to, conventional cerebral angiography in the management of cerebral aneurysms awaits further experience. In this study, we evaluated the role of CTA specifically for the pre-operative assessment and planning of cerebral aneurysm surgery.
Patients and Methods:
We reviewed the relevant neuroimaging of all patients treated at Dartmouth Hitchcock Medical Center between January, 2001 and December, 2004 with a diagnosis of cerebral aneurysm and diagnostic evaluation with both CTA and conventional digital subtraction angiography (DSA) using standard imaging protocols. 32 patients underwent both CTA and DSA during the study period for a total of 36 aneurysms. Images were independently re-assesed by two neurosurgeons for information valuable for pre-operative surgical planning.
Results:
In 26 of 36 aneurysms (72%), the CTA was felt to provide the best image quality in defining the morphology of the aneurysm. In 14 aneurysms (39%), CTA provided clinically valuable anatomic detail not demonstrated on DSA, largely due to better visualization of parent and perforating vessel relationships at the aneurysm neck. There were no instances where a lesion was seen on DSA but missed on CTA. The DSA was of most clinical value in determining flow dynamics, such as the arterial supply of an anterior communicating artery aneurysm and distal anterior cerebral branches via the two A1 segments.
Conclusion:
CTA with three-dimensional reconstructions is a valuable adjunct to the preoperative evaluation of cerebral aneurysms. We advocate routine use of CTA in all patients in whom surgical aneurysm repair is planned, even when DSA has already been performed.
INTRODUCTION
Computed tomographic angiography (CTA) has become increasingly utilized for the evaluation of cerebrovascular disease, particularly for intracranial aneurysms and aneurysmal subarachnoid hemorrhage [1-6]. CTA is relatively fast, minimally invasive, and requires less mobilization of resources compared with cerebral angiography. The routine use of CTA in the initial diagnostic management of aneurysmal subarachnoid hemorrhage has been well documented [2, 3, 7, 8]. Moreover, with improved hardware, software, and familiarity with the protocol, the sensitivity and specificity of CTA for detecting aneurysms has been reported to approach that of conventional intra-arterial digital subtraction angiography (DSA) [3, 7, 9, 10].
At our institution, we regularly use CTA as a first line diagnostic tool to evaluate subarachnoid hemorrhage. It is a modality that has been embraced by the treating clinicians and neuroradiologists. As our experience with CTA for evaluating and diagnosing aneurysms has grown, we have increasingly relied on the quality and resolution with which CTA can define the anatomy of cerebral aneurysms, and the ability to place it within the context of the adjacent vascular and bony anatomy. In our clinical experience, in multiple cases it seemed that the CTA three-dimensional reconstructions provided a superior image of the aneurysm compared to DSA, and also endowed the surgeon with additional useful information with respect to aneurysmal anatomy and relationships to parent and take-off vessels. Based on this experience, we hypothesized that CTA may provide clinically useful information for presurgical planning in aneurysm surgery even when conventional DSA imaging was already available.
METHODS
We reviewed all patients treated at Dartmouth Hitchcock Medical Center between January 2001 and December 2004 with a diagnosis of cerebral aneurysm and diagnostic evaluation with both CTA and DSA. Of the total number of patients identified with a diagnosis of cerebral aneurysm during the study period, 32 patients with 36 aneurysms had undergone diagnostic evaluation with both CTA and DSA. With the exception of the few studies from 2001, which were obtained with a 4 slice GE CT scanner, all CTA studies were performed using a General Electric Lightspeed ULTRA 16 slice helical CT scanner in the following protocol: anterior-posterior (AP) and lateral scout of head was initially obtained; IV contrast: 140cc Omnipaque 350 at 4-5 cc/second with a 15 second prep delay was injected through an 18 gauge needle; scan rostral from C-2 with 1.25mm thick slices every 0.6mm, 562:1 pitch, 5.62 speed (mm/ROT), 140 kV, 280 MA, helical acquisition, 1 second standard algorithm,16cm Field of View. Volume-rendered 3-dimentional image reformations were performed on a Vital Images Work Station using Vitrea 2 software with the Circle of Willis protocol. All DSAs were performed using either monoplanar or biplanar fluoroscopy in customized General Electric angiography suites, with digital GE acquisition software and standard orthogonal and oblique views as required. CTAs and DSAs were originally reviewed by staff neuroradiologists at Dartmouth-Hitchcock Medical Center at dedicated digital radiology work stations. The studies were then re-reviewed and compared independently by two neurosurgeons specifically for the purposes of this study. For each patient, the CTA and DSA were reviewed initially alone, then together in a side-by-side comparison, and analyzed for localization of the aneurysm(s), the overall morphology of the aneurysm(s), and identification of relevant anatomic information. This second review was compared with the reports from the original reads by the neuroradiologists for consistency and accuracy. In cases where the corresponding author (J.A.F) had operated on the patient, intra-operative findings were compared with the pre-operative images of the aneurysms. Specific attention was placed on which modality generated images most helpful for intra-operative anatomic findings. For calculations of sensitivities and specificities of CTA, a gold standard combining DSA and intra-operative findings was used.
RESULTS
The distribution of aneurysms is listed in Table 1. Fourteen of 32 patients (44%) presented with aneurysmal subarachnoid hemorrhage, while the remaining patients were unruptured aneurysms (56%). The unruptured aneurysms were either incidentally noted (34%) or presented with headache (19%). There was one case of an unruptured aneurysm, which presented secondary to a workup for progressive hemiparesis.
Distribution of Aneurysms Evaluated in this Study
| Aneurysm Location | # |
|---|---|
| MCA | 10 |
| Anterior Communicating Artery | 7 |
| Basilar Tip | 4 |
| PICA | 4 |
| Posterior Communicating Artery | 3 |
| ICA/Ophthalmic Artery | 3 |
| Vertebral Artery/VBJ | 2 |
| Anterior Choroidal Artery | 1 |
| Pericallosal Artery | 1 |
| Superior Cerebellar Artery | 1 |
| Total | 36 |
MCA = middle cerebral artery, PICA = posterior inferior cerebellar artery, ICA = internal carotid artery, VBJ = vertebrobasilar junction.
In twenty-six of thirty-six aneurysms (72%), the CTA was felt to provide the best image quality in defining the morphology of the aneurysm (Table 2). This was almost entirely due to the three-dimensional reformatted images of the CTA. In fact, of the seven studies in which CTA was not felt to have the better image quality, five did not have high-quality three-dimensional reformatted images available. Thus, in those studies in which the 3-D reformatted CTA was available, twenty-six out of thirty-one aneurysms (84%), CTA provided the superior image of the aneurysm preoperatively (Table 2).
Clinical Utility of CTA Compared with DSA
| Clinical Utility | # | % |
|---|---|---|
| Improved morphologic assessment of aneurysm | 26/36 | 72% |
| -when CTA with 3D reformations were available | 26/31 | 84% |
| Improved pre-operative anatomic information | 14/36 | 39% |
| -improved visualization of parent and perforating vessel relationships | 9/14 | 64% |
| -improved identification of daughter sac and lobular morphology | 5/14 | 36% |
| -improved analysis of relationship to brainstem and skull base | 3/14 | 21% |
In fourteen aneurysms (39%), the CTA provided clinically valuable anatomic detail not demonstrated on DSA (Table 2). In nine out of these fourteen aneurysms (64%), there was preoperative clinical utility in better visualization of parent and perforating vessel relationships at the aneurysm neck (Fig. 1). For example, in two cases of a posterior inferior cerebellar artery (PICA) aneurysm in our case series, the CTA provided clinically important information regarding the relationship of the aneurysm to the vertebral and basilar arteries, including the best representation of the take-off of the PICA from the aneurysm neck. In one case, the right vertebral artery was unable to be selectively catheterized for DSA (Fig. 2). The relationship of the aneurysm to the parent and perforating arteries was poorly demonstrated on the innominate vessel injection, but was clearly seen on CTA (Fig. 2). Furthermore, in five out of the fourteen aneurysms (36%), the CTA revealed markedly improved details of a daughter sac and of the lobular/saccular pattern of the aneurysm (Fig. 3). In addition, the relationship to the brainstem and skull base was better appreciated with CTA in three (21%) aneurysms (Fig. 4).

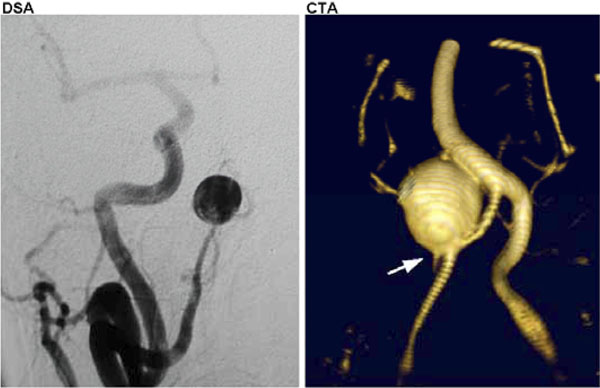
Right superior cerebellar artery aneurysm as visualized by conventional digital subtraction angiography (DSA, left) compared with the same aneurysm visualized by computerized tomographic angiography (CTA, right). Note the high degree of anatomic resolution and the imaging detail of the parent/daughter vessel relationships at the aneurysm neck.
Right posterior inferior cerebellar artery (PICA) aneurysm as imaged by DSA (left) and CTA (right). The right vertebral was not amenable to selective catheterization, and the subsequent innominate artery injection does not reveal much detail to the aneurysm and its vascular relationships compared with the result from the CTA. Note the relationship to the basilar artery, not seen with DSA, and PICA take-off at the base of the aneurysm (arrow) seen on CTA.
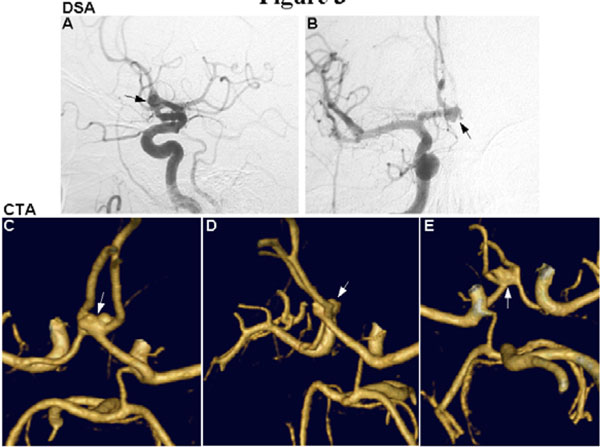
Anterior communicating artery aneurysm seen by DSA (A and B, arrows) and CTA (C-E, arrows). The lobular/saccular morphology of the aneurysm is more clearly seen on CTA, and the ability for rotational views allows visualization of the anatomy from above (A), the side (B), and below (C) to provide a valuable three-dimensional representation of the aneurysm.
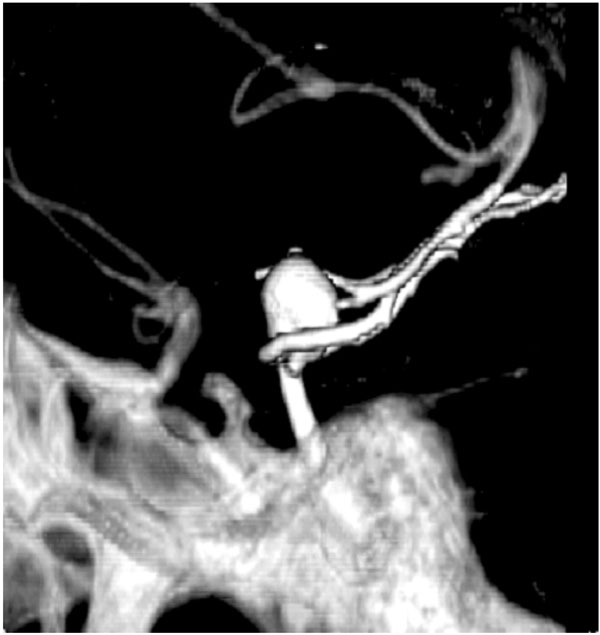
Aneurysm at the tip of the basilar artery seen on CTA. Aneurysm projection and relationship to the skull base bony anatomy is unambiguously seen.
In this subset of cases, there were no instances where an aneurysm was seen on DSA but not visualized on CTA. In fact, one aneurysm initially missed on angiography was demonstrated on subsequent CTA. This was a vertebrobasilar junction aneurysm at the base of a fusiform basilar artery dilation, and it was poorly appreciated on DSA due to a masking of the aneurysm along the parent vessel (Fig. 5A and C). The presence of the aneurysm was clearly demonstrated by CTA (Fig. 5B and D). This yielded a sensitivity and specificity for CTA of 100%, based on a gold standard set by DSA and intra-operative findings.
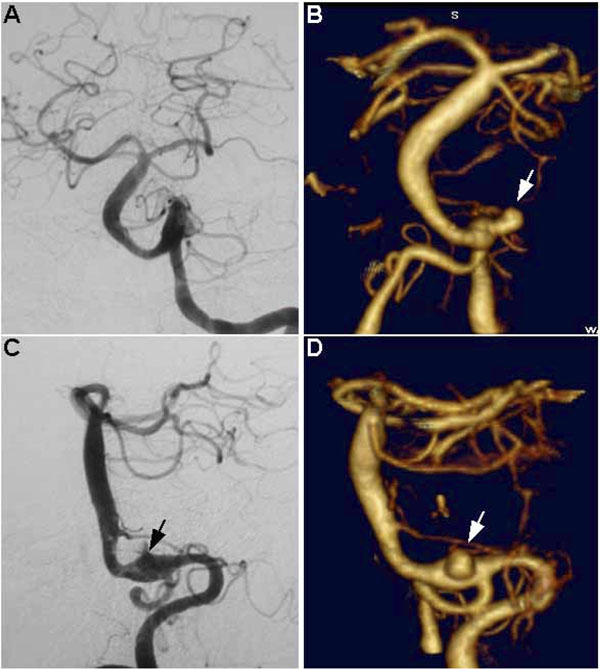
Fusiform basilar artery aneurysm with a saccular aneurysm at the left vertebrobasilar junction. Note how this aneurysm is poorly seen on DSA (A and C, arrow shows probable location) and was initially missed using this modality, whereas it is unequivocally visualized on CTA (B and D, arrows). A and B represent anterior-posterior (AP) views, C and D represent lateral views.
The DSA was felt to be of most clinical value in situations where physiologic arterial flow patterns were of importance preoperatively. For example, the identification of the arterial supply of an anterior communicating artery aneurysm and distal anterior cerebral branches via the two A1 segments was felt to be relevant to choosing the side of approach and for consideration in clip application. Another example in our series involved information regarding the relative contributions of the vertebral arteries to the basilar artery. This flow-related information is useful in planning the surgical approach to facilitate proximal control, as well as to estimate the risk of cerebral infarction due to temporary or permanent arterial occlusion.
DISCUSSION
There has been a substantial evolution in the relative roles of CTA compared with DSA for the diagnostic workup of cerebral aneurysms [2, 3]. We routinely obtain CTA as an initial diagnostic imaging test when suspecting a cerebral aneurysm, particularly in the setting of subarachnoid hemorrhage. In this situation, CTA provides several advantages over DSA: it is quick; it is relatively non-invasive; it involves less use of contrast dye compared with a four-vessel angiogram; it is relatively economic; and it involves mobilization of fewer personnel and resources.
As our experience with CTA has grown, we have been impressed by the image quality and degree of anatomic and vascular detail captured with this modality. In this study, we reevaluated the preoperative imaging characteristics of cerebral aneurysms in thirty-two consecutive patients at our institution diagnosed with a cerebral aneurysm, and who also had diagnostic workup including both CTA and DSA. Our intention was to see whether CTA provided any qualitative information, in addition to that obtained with DSA, which would be of clinical value for preoperative assessment. Our findings suggest that CTA with three-dimensional reformatted imaging provided useful clinical information not provided by DSA in 39% of cases, showing that it can be a valuable adjunct in the preoperative evaluation of cerebral aneurysms independent of conventional DSA. This advantage was mostly due to the additional information regarding parent vessel and perforator vessel relationships with the aneurysm, as well as the overall aneurysm morphology, projection, and relationship to the skull base. In our series, the utility of CTA in defining parent and perforator vessel relationships were most impressive with respect to the two aneurysms involving the PICA/vertebrobasilar junction. This type of relational anatomy would also be of potential clinical utility in identifying the anterior choroidal artery in preparation for clipping of aneurysms near this structure.
CTA, with its reformatting capability and ability to generate three-dimensional rotational projections, clearly illustrates the anatomy in a manner representative of intra-operative anatomic findings. Reports have noted the potential for CTA in pre-procedural planning [11-14]. CTA has been shown to be especially useful in particular anatomic locations, such as for certain posterior circulation aneurysms and for ophthalmic artery aneurysms [15-17]. In addition, some surgeons have used CTA for the evaluation of venous anatomy in preoperative planning [18, 19]. CTA can also allow for a potentially more precise procedure, as its relative ease of use with current frameless stereotactic systems has enabled keyhole craniotomies in distal MCA aneurysm clipping [20]. We, however, believe that CTA provides certain data with respect to relational anatomy that is an improvement over DSA with respect to preoperative planning, and, therefore, should be a routine part of pre-operative workup for aneurysm surgery.
Of note, we found that without the three-dimensional reformatted images, and the ability for subsequent rotational analysis of the aneurysm and adjacent anatomy, there was no substantial qualitative benefit to CTA over DSA for preoperative assessment. For instance, we have found paraclinoid lesions difficult to accurately reconstruct in three dimensions, and generally, only the two-dimensional reformatted images are of value. In the future, improved bone subtraction algorithms may help solve this issue. Currently, the skull base anatomy is well demonstrated in relation to the aneurysm by CTA, and the clinical utility of this remains to be seen. We have noted, however, that clipped, stented, and coiled lesions can be demonstrated surprisingly well on CTA source images as well as the two-dimensional reformatted images. This has allowed us to provide non-invasive follow-up for patients who have had endovascular therapies, and supports the utility of CTA aside from its three-dimensional reformation capabilities.
It should be stated that DSA data can also be reformatted to show three-dimensional images which have been used in pre-procedural planning [21]. In our view, CTA can create a similar, or arguably better, representation of many aneurysms and their surrounding anatomy without the inherent risks and additional time posed by DSA. In addition, there have been reports of three-dimensional MR angiography (MRA) for preoperative evaluation of cerebral aneurysms [22]. MR has the theoretical advantage of a non-invasive modality with the ability to provide quantitative flow information in addition to anatomic detail. It will be interesting to see whether improvements in MR technology will further advance cerebrovascular imaging; however, we feel that with the current technology in use, CTA provides a better image, and does not have the lengthy acquisition time and encumbrances associated with MRI, particularly when dealing with sick, intubated patients.
In this series, CTA did not miss any lesions seen on DSA, and in fact, revealed an obvious aneurysm that was initially missed on DSA. Using a combination of DSA and intraoperative findings as the gold standard, this yielded a sensitivity and specificity of 100% for CTA. Although in our limited subset of patients, no lesions were missed on CTA, larger series have shown a small false negative rate compared to DSA, generally with small <3-4mm aneurysms [2, 9, 10, 23]. This would certainly need to be taken into account in a situation where workup for subarachnoid hemorrhage by CTA is negative, or in cases where there is a high index of suspicion for an aneurysm or other vascular lesion. We continue to employ DSA in these instances.
In conclusion, our results show that CTA can indeed provide additional information of clinical value in many cases when compared to DSA. While most neuroradiologists may be aware of the power of CTA in this regard, neurosurgeons may not routinely obtain a CTA for anatomic pre-procedural planning. We feel this information is advantageous, when available, for preoperative planning for aneurysm surgery, and we therefore advocate routine use of CTA in all patients in whom surgical aneurysm repair is planned, even when DSA has already been performed.


