All published articles of this journal are available on ScienceDirect.
Changes in Immunological Parameters and Neurotrophic Factors in Patients with Compression, Post-traumatic Non-gunshot and Gunshot Neuropathies and Plexopathies
Abstract
Introduction:
Chronic pain, which usually occurs after traumatic damage to nerves and plexuses, is an additional burden on the patient's quality of life, which is already impaired due to motor, sensory and autonomic deficits. The aim is to study and clarify changes in immunological parameters and neurotrophic factors in patients with compression, post-traumatic non-gunshot and gunshot neuropathies and plexopathies.
Materials and Methods:
The study included 93 men aged 21 to 59 with neuropathy and plexopathy of traumatic and non-traumatic origin, divided into 3 groups. Patients underwent clinical and neurological examination starting at the onset of clinical manifestations and in the dynamics, electromyography, and ultrasound. The immunological examination was performed from 12 to 24 months, starting at the onset of the disease.
Results:
Differences between the study groups in the concentration of circulating immune complexes and lymphocytotoxicity and decrease in CD4 + T lymphocytes were statistically significant. In the NBT-test results, which characterize the difference between spontaneous and zymosan-stimulated oxygen-dependent phagocytosis, it was found that the cells oxidized granules of nitroblue tetrazolium dye with different degrees of intensity, depending on the regenerative capacity of enzymes. We found an increase in the quantitative content of the beta-NGF 303 [35.2; 504.5] pg/ml in the blood of patients of group III with post-traumatic gunshot neuropathies and plexopathies accompanied by chronic neuropathic pain syndrome at a statistically significant level (Kruskal-Wallis test, p=0.0181).
Conclusion:
The study showed a statistically significant (p<0.05) violations of the regulatory link of immune system in patients with post-traumatic gunshot neuropathies and plexopathies, increased circulating immune complexes level, disorders of oxygen-dependent and oxygen-independent phagocytosis, reduced enzymatic regenerative capacity, significant increase in beta-NGF, Thus, as a result of the study, we found statistically significant (p<0.05) violations of the regulatory link of immune system in patients with post-traumatic gunshot neuropathies and plexopathies, increased circulating immune complexes level, disorders of oxygen-dependent and oxygen-independent phagocytosis, reduced enzymatic regenerative capacity, significant increase in beta-NGF 303 [35.2; 504.5] pg / ml, indicating the ongoing process of chronic inflammation, stimulation of the sympathetic nervous system and sensory fibers, which leads to the maintenance and severity of chronic neuropathic pain even after 12 months or more since the injury with damage to nerves and plexuses of the limbs.
1. INTRODUCTION
Chronic pain, which usually occurs after traumatic damage to nerves and plexuses, is an additional burden on the patient's quality of life, which is already impaired due to motor, sensory and autonomic deficits [1]. It has been recognized that interactions between the nervous and immune systems play an important role in initiating and maintaining pain. In addition to changes in neuronal function, current data suggest a two-way link with the immune system in chronic pain initiation and maintenance [2]. Mutual signaling between immunocompetent cells in the central nervous system has become a key phenomenon underlying the mechanisms of chronic pain. Nervous excitability can be greatly enhanced by classical neurotransmitters and immune mediators released from the CNS-resident microglia and astrocytes, as well as from infiltrating cells such as T-cells [3]. Phagocytosis is necessary to maintain tissue homeostasis in many inflammatory and autoimmune diseases, but its role in the pathogenesis of the peripheral and central nervous systems’ diseases has been studied insufficiently. Phagocytes are universal effectors of homeostasis, which respond to numerous signals arising from the destabilization of the internal environment. Based on the degree of changes in phagocytic reactions, it is possible, on the one hand, to evaluate the reserves of the immune response, and on the other hand - to determine the intensity and dynamics of infectious or non-infectious pathological processes [4, 5].
There is growing evidence of the neurotrophic factors’ contribution to the physiology and pathology of the nervous system. Neurotrophic factors stimulate the survival, migration, proliferation, and regeneration of neurons and affect the mechanisms of neuroplasticity by regulating the formation and plasticity of new synapses, sprouting (axon growth), and arborization (dendritic branching) in the direction of cells-targets, the activity of ion channels and neurotransmitter receptors affect the processes of myelination and remyelination [6]. The properties of neurotrophins are related to the ability to prevent the formation of free radicals, impede oxidative stress and affect apoptosis, as well as monitor the preservation of structural and functional integrity of neurons and the processes of their physiological development [7]. Nerve growth factor (NGF) became the first known neurotrophic factor. It regulates the survival of sympathetic and sensory neurons of the peripheral nervous system, as well as cholinergic neurons of the CNS [8]. In addition, NGF affects the release of mediators (acetylcholine, glutamate, etc.) in neuromuscular synapses and synaptosomes of the hippocampus [9]. NGF plays a vital role in neuropathic pain and is mainly synthesized and released by nonneuronal cells in the periphery compared with those of the spinal cord after damage to peripheral nerves [10]. NT-3 plays a role in the development of the sympathetic nervous system. Targets for NT-3 are cholinergic neurons of the frontal lobe, cholinergic neurons of the hypothalamus, neurons of the cerebral cortex, proprioceptive system, muscle fibers, neurons of the auditory nuclei, and neuroglial cells [11]. Thus, further study of changes in neurotrophic factors is an important issue that can deepen the knowledge of the pathogenesis, course of the disease and neuroregenerative processes of damaged nerves and plexuses, thereby providing an opportunity to influence the mechanisms of impaired function recovery [12].
The aim is to study and clarify changes in immunological parameters and neurotrophic factors in patients with compression, post-traumatic non-gunshot and gunshot neuropathies and plexopathies.
2. MATERIALS AND METHODS
2.1. Inclusion Criteria
The study included 93 men aged 21 to 59 with neuropathy and plexopathy of traumatic and non-traumatic origin, divided into 3 groups. Group I included 30 patients with compression neuropathy and plexopathy, out of which neuropathies were 25 and plexopathies were 5. Group II included 30 patients with post-traumatic non-gunshot neuropathy and plexopathy due to fractures of the upper and lower limbs, joints dislocations, and injuries caused by knives, glass, cutting and sharp tools, due to soft tissue slaughter, out of which neuropathies - 26, plexopathies - 4. Group III included 33 patients with post-traumatic gunshot neuropathy and plexopathy (11 bullet and 22 shrapnel wounds of the limbs), out of which neuropathies were 27 and plexopathies were 6.
2.2. Exclusion Criteria
Patients were excluded from the study if diagnosed with chronic viral and bacterial infections, central nervous system diseases, or autoimmune, oncological and hematological disorders. Scales and questionnaires assessed patients with compression neuropathy and plexopathy to detect chronic neuropathic pain. If patients with compression neuropathy and plexopathy were diagnosed with chronic neuropathic pain, they were excluded from the study.
2.3. Methods
This was a prospective, observational study of patients hospitalized due to neuropathies and plexopathies at Military Medical Clinical Center, Kharkiv, Ukraine, from 2015 to 2021. Patients underwent clinical and neurological examination starting at the onset of clinical manifestations and in the dynamics, electromyography, and ultrasound. Pain scales (VAS) and questionnaires (DN 4, Pain Detect) were used to determine the pain syndrome. The immunological examination was performed from 12 to 24 months, starting at the onset of the disease. Cellular and humoral immunity of peripheral blood were evaluated to study the immunological status of all patients. Informed consent was obtained from all subjects who were involved in the study. The study was approved by the Academic Board of Kharkiv Medical Academy of Postgraduate Education (protocol # 9 by November 13, 2019) as a part of a larger study. This research was conducted on humans per the Helsinki Declaration of 1975, revised in 2013.
2.3.1. Method for Determining the Expression of CD3+, CD4+, CD8+ Differentiation Clusters on T-lymphocyte Subpopulations
An immunofluorescence method using FITC-labeled monoclonal antibodies was applied to assess the expression of CD3 + CD4 + CD8 + differentiation clusters in T- and B-lymphocyte subpopulations. The immunoregulatory index (IRI) was calculated using the ratio CD4 + / CD8 +.
2.3.2. Method of Determining the Phagocytic Activity of Granulocytic Neutrophils
The phagocytic activity of neutrophils was studied in leukocyte suspension extracted from heparinized blood. For the study, a leukocyte suspension and a washed suspension of Saccharomyces cerevisiae were combined as a 1:1 ratio. The studied samples were incubated for 1 hour at 37°C with regular stirring. After incubation, the resulting smears were stained with Romanovsky's dye, followed by microscopic examination [13].
2.3.3. Method of Studying Oxygen-dependent Metabolism of Neutrophil Granulocytes
Using the method of A.N. Mayansky, we studied the oxygen-dependent metabolism of neutrophilic granulocytes in the reaction of spontaneous and stimulated reduction of nitroblue tetrazolium (NBT-test). During the process, NBT is reduced to the undissolvable diformazan, which is determined in cells in the form of blue granules, which are visualized under microscopy [14]. Average cytochemical coefficients in spontaneous (ACK spont) and stimulated (ACK stim) tests were calculated according to the Astaldi-Verg formula. The stimulation index (SI) was calculated as follows: SI = (Percentage of positive cells in NBT-test spontaneous) / (Percentage of positive cells in NBT-test stimulated) [15].
2.3.4. Method of Determining the Concentration of Circulating Immune Complexes
The serum level of circulating immune complexes was evaluated spectrophotometrically after incubating samples in borate buffer and polyethylene glycol (PEG) at 22°C. During the incubation, the circulating immune complexes were precipitated on polyethylene glycol, which was reflected in the change in the optical density of the samples. The optical density measurements were performed spectrophotometrically.
2.3.5. Method of Determining the Circulating Immune Complexes Constant
The principle of determining the CIC constant is based on the selective precipitation of CIC in the PEG density gradient. After applying different amounts (1.5 ml and 2.0 ml) of a 5% solution of PEG 6,000 (sample 1 and sample 2, respectively) and 18 hours at 4°C. After centrifugation at 2,200 rpm for 17 minutes, the supernatant liquid was separated. The supernatant liquid was diluted with 0.1 N sodium hydroxide, and the optical density at a wavelength of λ = 280 nm was determined spectrophotometrically against sodium hydroxide. The CIC constant was calculated using the formula: CIC k = D2/ D1, where D1 is the optical density in sample 1; D2 is the optical density in sample 2.
2.3.6. Test for Lymphocytotoxicity According to the Terasaki Method
Determination of the content of membranotropic cytotoxic factors was performed using the Terasaki method [16]. A leukocyte suspension, prepared from heparinized blood and incubated with homologous serum at 37°C for 30 minutes, was used to perform the test. A complement was added drop by drop to form an antigen-complement-antibody complex. After incubation at 37°C for 15 minutes, dye eosin and methylene blue were added to the suspension of the cells with a damaged cell membrane. Cells were counted using an Ergoval light microscope based on differential staining. The level of membranotropic cytotoxic factors was estimated by the percentage ratio of living and dead cells.
2.3.7. Method of Quantitative Determination of Neurotrophic Factors in Blood Serum
Quantitative evaluation of the beta subunit of human nerve growth factor (beta-NGF) in serum was performed by enzyme-linked immunosorbent assay using the Ray Bio Human Beta-NGF ELISA Kit.
Quantitative evaluation of neurotrophin-3 (NT-3) in serum was performed by enzyme-linked immunosorbent assay using the Ray Bio Human NT-3 ELISA Kit.
Non-heparinized peripheral blood, taken from the elbow vein under sterile conditions in 5 ml, was used for serum testing. The samples were centrifuged at 3000 rpm at 22°C, frozen at a temperature not lower than -20°C, and stored for up to 3-6 months to obtain serum. The method is based on specific antibodies to human beta-NGF and NT-3, which are absorbed in the wells of the microtablet. Standards and samples were introduced into the wells of the tablet, where beta-NGF and NT-3 that were present in the sample bounded to the immobilized in the wells antibodies. Biotinylated antibodies to beta-NGF and NT-3 were added after incubation and washing. After the incubation and washing, biotinylated antibodies were removed, and the streptavidin-HRP conjugate was added to the wells. After incubation and washing, TMB substrate was added to the wells, and the staining developed proportionally to the amount of bound human beta-NGF and NT-3. A stop reagent was used to stop the reaction, changing the solution's color from blue to yellow. The concentration was measured at a wavelength of λ = 450 nm.
2.4. Data Analysis
Data processing and analysis were performed using STATISTICA 6.0 (StatSoftInc., USA) and SPSS 27.0 (IBM, USA). Due to the sample size, quantitative comparisons were performed by non-parametric methods using the Kruskal-Wallis test and the median test. Quantitative variables are presented as the mean value with standard deviation (M ± SD) in the case of normal distribution and the median with an interquartile range of 25% - 75% (Me [Q1; Q3]) for variables with abnormal distribution; qualitative variables - in the form of numerical values with a percentage of the total. Differences at p<0.05 were considered statistically significant.
3. RESULTS
In group I, the age of patients was 44 (37; 49) years; damage of nerves and plexuses of the upper limbs was observed in 76.6%, and lower limbs - in 23.3% of cases. In group II, the age of patients was 38.5 (32; 46) years; damage of nerves and plexuses of the upper limbs was observed in 63.3%, and lower limbs - in 36.6% of cases. The age of patients in group III was 42 (36; 47) years; damage of nerves and plexuses of the upper limbs was observed in 60.6%, and lower limbs - in 39.4% of cases.
In all groups without significant difference (p>0.05), the typical neurological disorders were motor disorders, which manifested in peripheral paresis or plegia of the corresponding muscle group and were accompanied by a decrease or loss of tendon and periosteal reflexes. Positive and negative symptoms represented sensitivity disorders. Negative symptoms included anesthesia and hypoaesthesia in the innervation of the limbs’ nerves. Positive symptoms in patients were represented by allodynia, hyperalgesia, hyperpathy, dysesthesia, and hyperesthesia. Since pain is more common in partial nerve damage with incomplete interruption of the nerve fibers - the positive symptoms were of particular interest.
In the study of oxygen-independent phagocytosis in the groups shown in Table 1, the number of cells that entered phagocytosis did not exceed the reference values in all study groups. Patients of group I with compression neuropathies and plexopathies showed an increase in the absorption and sufficient digestive capacity of phagocytes, indicating the absence of a defect of lysosomal enzymes caused by genetic defects.
Patients of group II with post-traumatic non-gunshot neuropathies and plexopathies showed an increase in absorption and a decrease in the digestive capacity of phagocytes. The greatest decrease in the digestive capacity of phagocytes was observed in patients of group III, which was statistically significant in the intergroup comparison (p<0.05), also in this group, there was a high absorption capacity, indicating a lack of phagocytosis in patients with post-traumatic gunshot neuropathy and plexopathy accompanied by chronic neuropathic pain syndrome.
In the NBT-test results, which characterize the difference between spontaneous and zymosan-stimulated oxygen-dependent phagocytosis, it was found that the cells oxidized granules of nitroblue tetrazolium dye with different degrees of intensity, depending on the regenerative capacity of enzymes. Spontaneous levels of the oxidative capacity of enzymes in all groups exceeded the reference values and had statistical significance (p<0.05) in the intergroup comparison, with the greatest expression in patients of group III.
Due to the lack of sufficient difference between spontaneous and stimulated tests, the stimulation index was significantly lower than the reference values, as shown in Table 2. In groups II and III, there was a higher difference between spontaneous and stimulated oxidation of antigens due to the active forms of oxygen than in group I. Statistically significant violation of oxygen-dependent phagocytosis was found in all study groups of patients with neuropathy and plexopathy, which indicates a lack of NADPH oxidase system due to depletion of the oxidative reserve of neutrophils. The stimulation index indicated the integrated bactericidal activity of phagocytes and was significantly reduced in all subjects, with the greatest severity in group I, indicating chronic antigenic load.
As shown in Table 3, the concentration of circulating immune complexes (CICs) in all patients of the three groups was higher than the reference values. Differences between study groups in the concentration of circulating immune complexes and lymphocytotoxicity were statistically significant.
| Indexes | Phagocytic Index (PI),% | Phagocytic Number (PN) | Completeness Index of Phagocytosis (CIP) |
|---|---|---|---|
| Reference values | 70-90 | 2.5-4 | 1.1-1.22 |
| Group І | 85 (82;90) | 4.18 (3.88;4.85) | 1.15 (0.95;1.25)* |
| Group ІІ | 87 (85;92) | 4.175 (3.98;4.53) | 1.05 (0.92;1.15)* |
| Group ІІI | 87 (85;90) | 4.33 (3.75;4.87) | 1.01 (0.9;1.08)* |
| Indexes |
Percentage of Positive Cells in NBT Test Spontaneous |
Percentage of Positive Cells in NBT Test Stimulated |
Average Cytochemical Coefficient (ACK Spont) NBT Test Spontaneous |
Average Cytochemical Coefficient in (ACK Stim) NBT Test Stimulated |
Stimulation Index (SI) |
|---|---|---|---|---|---|
| Reference values | 10.1 | 65.2 | 1.3 | 1.3 | 7.1 |
| Group І | 41 (30;48)* | 42 (32;46)* | 0.6 (0.4;0.72)* | 0.6 (0.52;0.68)* | 1.1 (0.83;1.29)* |
| Group ІІ | 39 (29;42)* | 58 (41;68)* | 0.57 (0.44;0.63)* | 0.87 (0.64;1.09)* | 1.67 (1.08;2.08)* |
| Group ІІI | 43 (37;51)* | 57 (52;70)* | 0.67 (0.63;0.74)* | 0.96 (0.7;1.1)* | 1.37 (1.19;1.52)* |
| Indexes | Concentration of CICs | Constanta of CICs | Lymphocytotoxicity,% |
|---|---|---|---|
| Reference values | 50-100 | 1.1-1.5 | 10-30 |
| Group І | 123 (109;138)* | 1.0 (0.95;1.1) | 47 (42;50)* |
| Group ІІ | 110 (88;123)* | 1.0 (0.9;1.1) | 42 (40;43)* |
| Group ІІI | 127 (112;148)* | 1.0 (0.95;1.1) | 45 (42;50)* |
The maximum concentration of circulating immune complexes was found in patients of group III, combined with a decrease in the circulating immune complexes constant. The obtained data indicate high pathogenicity of circulating immune complexes and additional tissue alteration due to insufficient clearance in patients with post-traumatic gunshot neuropathy and plexopathy accompanied by chronic neuropathic pain syndrome. The content of cytotoxic factors was significantly increased in all examined groups, with the highest rate in patients of group I – the lymphocytotoxicity was 47 (42; 50) % and in group III the lymphocytotoxicity was 45 (42; 50) %, in group II the lymphocytotoxicity was 42 (40; 43) %.
In the study of cellular immunity in all groups, there is a decrease in CD4 + T lymphocytes (Fig. 1) compared to reference values; in the intergroup comparison, a statistically significant difference was found in patients of groups II and III (Median test, p=0.0205). In group I, CD4 + T lymphocytes were 29.5 (25; 32) %, in group II - 27 (25; 29) % and group III - 27 (22; 34) %, which indicates more pronounced violations of the regulatory link of immunity in patients with traumatic damage to nerves and plexuses of the limbs.
In the study of the quantitative content of NT-3 in the blood (Fig. 2) in patients of group I with compression neuropathy and plexopathy, the level of NT-3 was 11.1 (8.2; 13.2) pg/ml, in patients of group II with post-traumatic non-gunshot neuropathy and plexopathy - 10.05 (7.8; 26.2) pg/ml, in patients of group III with post-traumatic gunshot neuropathy and plexopathy accompanied by chronic neuropathic pain the level of NT-3 was 9 (6.8; 14.8) pg/ml. No statistically significant differences were found in the intergroup comparison.
According to the research results, we found an increase in the quantitative content of beta-NGF (Fig. 3) in the blood of patients of group III with post-traumatic gunshot neuropathies and plexopathies accompanied by chronic neuropathic pain syndrome at a statistically significant level (Kruskal-Wallis test, p=0.0181). In group I patients with compression neuropathies and plexopathies, the level of beta-NGF was 83.6 (42; 335.4) pg/ml; in group II patients with post-traumatic non-gunshot neuropathy and plexopathy - 64.65 (25; 97.5) pg/ml, in patients of group III the level of beta-NGF was 303 (35.2; 504.5) pg/ml, indicating an ongoing process of chronic inflammation, stimulation of the sympathetic nervous system and sensory fibers, which determines the maintenance and severity of chronic neuropathic pain in patients with post-traumatic gunshot neuropathies and plexopathies.
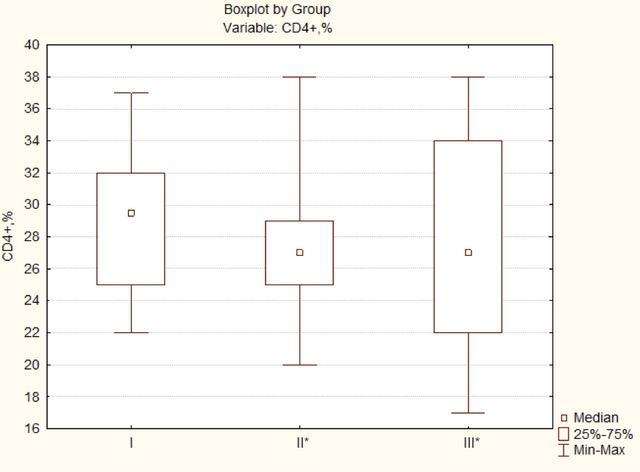
Differences in the intergroup comparison are significant at p<0.05.
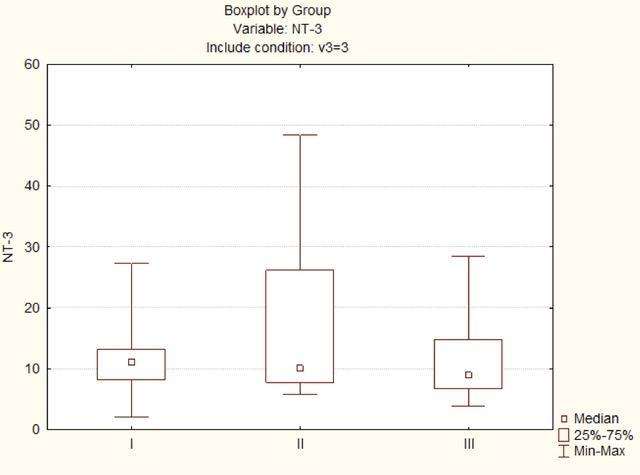
Differences in the intergroup comparison are significant at p<0.05.
Differences in the intergroup comparison are significant at p<0.05.
4. DISCUSSION
Tsirkin et al. [17] described that at a certain stage of the inflammatory process, effector T-helpers, whose main function is to enhance the adaptive immune response, synthesize proinflammatory cytokines that increase the production of neurotrophins. The increased Nerve growth factor is accompanied by activation of the Th2 subpopulation, which promotes the synthesis of antibodies by plasma cells on the background of the interleukin-4 secretion initiation. Since there is a mutual suppressive effect of Th2 on Th1, the activation of antibody formation under the influence of cytokines synthesized by the Th2 subpopulation is determined. At the first stage of the inflammatory process, there is an active synthesis of specific paraproteins of the IgM class. Active synthesis of IgM antibodies that can actively affect the myelin of nerve fibers leads to specific nerve damage [17]. Our study also found that the content of the circulating immune complexes was maximal in patients with post-traumatic gunshot neuropathies and plexopathies due to high antibody production and differentiation of T-helpers towards T-helper 2. Activation of T-helper 2 was accompanied by increased secretion of beta-NGF. In patients with post-traumatic non-gunshot neuropathy and plexopathy, antibody production was also high due to T-helper 2 activation. This indicates that under the traumatic injuries of nerves and plexuses of the limbs, immune disorders have a protracted course due to the long-term support of nerve fiber damage, which is one of the mechanisms of the formation of chronic neuropathic pain.
Ulyanov et al. noted that phagocytes initiate tissue regeneration, including nerve, on the principle of balance between catabolic and anabolic processes by activating nerve growth factors, which is an essential condition for maintaining cellular homeostasis of neurons, processes of remyelination of axons and minimal compensation of lost functions in the post-traumatic period. The movement of leukocytes to the foci of inflammation occurs on the concentration gradient of some endogenous and exogenous substances in plasma and exudate. Some microorganisms can synthesize a chemotaxis inhibitor, which leads to a more severe inflammatory process. The attraction of phagocytes occurs due to the opsonizing effect of plasma under joining the antigen of humoral factors, such as immunoglobulins, with the formation of circulating immune complexes. Without opsonins, phagocytosis is less effective or does not occur at all. Endocytosis is intracellular digestion provided by two processes: oxygen-independent and oxygen-dependent phagocytosis. Intracellular transformations of the absorbed antigen during phagocytosis are connected to a fusion of a phagosome with a lysosome and the formation of the phagolysosome filled with hydrolytic enzymes. Hydrolytic enzymes provide antigen degradation. Depending on the results of intracellular digestion, phagocytosis may be complete or incomplete if the microorganisms remain viable. The second mechanism of phagocytosis is oxygen-dependent, which is associated with the production of active oxygen metabolites that are powerful bactericidal substances. Phagocytes usually use anaerobic glycolysis, but they absorb more oxygen in this case, leading to a respiratory explosion involving NADPH oxidase. Increased generation of oxygen metabolites by phagocytes and excessive and inadequate release into the extracellular space in non-physiological concentrations have a negative side because they can cause tissue destruction, both in the inflammatory foci and remotely [18]. In our study, we also found the activation of the beta-NGF in response to the harmful effects of oxidative stress by increased spontaneous activity of NADPH enzymes in oxygen-dependent phagocytosis, which was the highest in patients with posttraumatic gunshot neuropathies and plexopathies.
In all studied groups, patients received vitamin therapy, nonsteroidal anti-inflammatory drugs, cholinesterase inhibitors, peripheral vasodilators and vasoprotectors during primary inpatient treatment. During further treatment, vitamins, cholinesterase inhibitors, peripheral vasodilators and vasoprotectors, nucleotides - cytidine monophosphate and uridine triphosphate, anticonvulsants for chronic neuropathic pain were prescribed. 30 days before the immunological examination, complex medical therapy was not carried out; patients were allowed to use anticonvulsants episodically, namely pregabalin 75 mg and gabapentin 300 mg for severe neuropathic pain. All drugs were excluded 48 hours before the immunological examination. For severe pain, an episodic use of anticonvulsants was allowed, and therefore we do not indicate whether immunological parameters would be more altered in the absence of correction of neuropathic pain over a long period. This will be addressed in future studies subjecting to consent and the physical capacity of our patients. However, in all studied groups, immunoprotective, immunomodulating therapy, and cytostatics were not prescribed or used for the entire observation period.
CONCLUSION
Maximum cytotoxicity was observed in patients with post-traumatic gunshot neuropathies and plexopathies with activation of the regulatory role of the Th2 subpopulation of lymphocytes. The phagocytosis completion index was below the reference values and increased NADPH reactions reduced the oxygen reserve in oxygen-dependent phagocytosis. Nonconformity of damage and compensatory capabilities of homeostasis in patients with post-traumatic gunshot neuropathies and plexopathies leads to clinically significant consequences.
Activation of the beta-NGF occurs in response to the harmful effects of oxidative stress due to increased spontaneous activity of NADPH enzymes in oxygen-dependent phagocytosis, which is the highest in patients of group III on the background of the reduced regenerative capacity of phagocyte enzymes. As for the changes in immunoreactivity in oxygen-independent phagocytosis, patients with post-traumatic neuropathy and plexopathy showed the greatest inhibition of the adhesive and digestive capacity of phagocytes. An increase in the beta-NGF factor was observed in the majority of patients in group III on the background of a significant increase in the CD4+ T lymphocyte subpopulation compared to the reference values and content of this population in groups I and II. NT-3 factor, which ensures the development, “maturation,” and myelination of actively regenerating damaged nerves in patients of group III, is suppressed compared to group II, but its concentration did not differ significantly from that in patients with nerve and plexus pathology of various origins.
Thus, as a result of the study, we found statistically significant (p<0.05) violations of the regulatory link of the immune system in patients with post-traumatic gunshot neuropathies and plexopathies, increased circulating immune complexes levels, disorders of oxygen-dependent and oxygen-independent phagocytosis, reduced enzymatic regenerative capacity, a significant increase in beta-NGF 303 [35.2; 504.5] pg/ml, indicating the ongoing process of chronic inflammation, stimulation of the sympathetic nervous system and sensory fibers, which leads to the maintenance and severity of chronic neuropathic pain even after 12 months or more since the injury with damage to nerves and plexuses of the limbs.
Identifying the features of immune and neurotrophic control disorders, their relationship and studying the role in the pathogenesis of chronic neuropathic pain in post-traumatic gunshot neuropathies and plexopathies deepen the understanding of the feedback of the immune and nervous systems formed in patients with traumatic injuries of nerves and plexuses of the limbs.
Further study of patterns, status, the interaction of immune and nervous systems, features and aspects of pathological processes may be a key point in developing comprehensive treatment programs with immunocorrective therapy depending on the identified immunological disorders to improve nerve fiber regeneration and reduce chronic neuropathic pain.
LIST OF ABBREVIATIONS
| ACK spont | = Average Cytochemical Coefficient NBT test spontaneous |
| ACK stim | = Average Cytochemical Coefficient in NBT test stimulated |
| CIC | = Circulating Immune Complexes |
| CIP | = Completeness Index of Phagocytosis |
| CNS | = Central Nervous System |
| NADPH | = Nicotinamide Adenine Dinucleotide Phosphate |
| NBT-test | = Nitro Blue Tetrazolium test |
| NGF | = Nerve Growth Factor |
| NT-3 | = Neurotrophin-3 |
| PEG | = Polyethylene Glycol |
| PI | = Phagocytic Index |
| PN | = Phagocytic Number |
| SI | = Stimulation Index |
AUTHOR'S CONTRIBUTION
Conceptualization, O.B., T.L. and O.K.; methodology, O.B. and O.K., L.D.; software, O.B. and L.D.; validation, O.B. and T.L.; formal analysis, O.B.; investigation, O.B.; resources, O.B. and L.D.; data curation, O.B., O.K.; writing—original draft preparation, O.B.; writing—review and editing, O.B, T.L. and O.K.; visualization, O.B.; supervision, O.B., T.L.; project administration, O.B. All authors have read and agreed to the published version of the manuscript.
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
The study was approved by the Academic Board of Kharkiv Medical Academy of Postgraduate Education (protocol # 9 by November 13, 2019) as a part of a larger study.
HUMAN AND ANIMAL RIGHTS
No animals were used in this research. All procedures performed in studies involving human participants were per the ethical standards of institutional and/or research committees and with the 1975 Declaration of Helsinki, as revised in 2013.
CONSENT FOR PUBLICATION
Informed consent was obtained from all participants.
STANDARDS FOR REPORTING
STROBE guidelines and methodology were followed.
AVAILABILITY OF DATA AND MATERIALS
All the data supporting this research's findings are available from the corresponding author [O.B] upon reasonable request.
FUNDING
None.
CONFLICT OF INTEREST
The authors declare no conflict of interest, financial or otherwise.
ACKNOWLEDGEMENTS
Declared none.


