All published articles of this journal are available on ScienceDirect.
Guillain-Barré Syndrome with Lethal Outcome Following COVID-19 Vaccination - Case Report Supported by Autopsy Examination
Abstract
Objective:
After the outbreak of the global pandemic caused by SARS-CoV-2 infection at the end of the year 2019, it took one year to start vaccination against this infection with products from various manufacturers. As of November 2021, more than 8 billion vaccine doses against COVID-19 have been administered, which is essentially linked to a spike in adverse events reports following these vaccinations, including a number of neurological adverse events.
Case Report:
We report a case of a 71-year-old patient with lethal fulminant onset of Guillain-Barré syndrome after the second dose of mRNA vaccine tozinameran. This is, to our best knowledge, the first case report of this adverse event supported by autopsy and histological examination. The patient presented with progressive ascending weakness and paresthesia, with typical cytoalbuminologic dissociation in cerebrospinal fluid and severe motoric and sensitive axonal-demyelinating polyneuropathy on electromyography. The patient’s history and complex diagnostic workup did not reveal any other possible causative factors. The patient did not respond to the treatment with intravenous immunoglobulins and died 10 days later due to aspiration bronchopneumonia as a complication of respiratory muscles paralysis.
Conclusion:
Most of the reported adverse reactions following COVID-19 vaccination include mild or moderate events noticed in the post-vaccination period; however, reports of possible lethal outcomes are no exception. Still, the overall incidence of GBS after vaccination does not significantly exceed its incidence in the general population. Each such report should be carefully examined by a team of specialists to prevent overestimation of lethal adverse events linked to vaccinations, especially in fatalities that happen in the post-vaccination period.
1. INTRODUCTION
Guillain-Barré syndrome (GBS) is a potentially life-threatening autoimmune disorder presenting with a spectrum of neurological symptoms. A typical clinical picture of GBS includes acute onset of sensory-motor ascending neuropathy [1]. Currently recognized different subtypes result from the immune attack on different components of the peripheral nervous system, including myelin sheets, Schwan cells, or axolemma [2]. The incidence of GBS is estimated to be between 0.62 – 2.66, which increases with age, more commonly affecting males [3]. Although GBS is relatively rare, it is recognized as the most common cause of acute postinfectious flaccid paralysis, so early detection and timely management of patients are of great importance.
Pathogens associated with the development of GBS include various bacteria and viruses; most recently, the development of GBS was noticed following COVID-19 infection [4, 5]. Campylobacter jejuni is so far considered the most common causative agent, with an estimation of 30 – 40% of GBS patients suffering from this infection shortly before the onset of first symptoms [6, 7]. Less commonly, GBS has been reported in association with non-infectious factors, such as surgical interventions [8-10], intake of certain drugs [11-13], or vaccinations.
The association of GBS with vaccination has been an ongoing discussion. A causative link has been so far established only with strain A/NewJersey/76 influenza vaccine [14] and rabies vaccine cultured in neural tissues [15]. Sporadic case studies following different vaccines continue to emerge, including vaccinations against seasonal influenza [16], H1N1 [17], hepatitis B [18], human papillomavirus (HPV) [19], and meningococcal vaccine [20].
With the COVID-19 pandemic, new vaccines have been introduced to the general population. As of October 2021, WHO approved several vector-based vaccines, inactivated virus vaccines, and mRNA-based vaccines [21]. Although rare, several severe nervous system disorders following these vaccines have been reported, including Bell’s palsy, stroke, acute disseminated encephalomyelitis, transverse myelitis, as well as Guillain-Barré syndrome [22, 23]. Here, we present a case of an acute onset of GBS following mRNA vaccination against COVID-19 with a lethal outcome. The diagnosis was confirmed by a post-mortal examination conducted by Slovak Health Care Surveillance Authority and evaluated by Slovak State Institute for Drug Control as a probable adverse event following vaccination, based on WHO-UMC (Uppsala Monitoring Center) system for standardized case causality assessment.
2. CASE PRESENTATION
A 71-year-old male patient with a history of chronic obstructive lung disease (GOLD A) and lung emphysema was vaccinated with the second dose of mRNA-based vaccine against COVID-19 (tozinameran). The next day, the patient observed increased temperature (38.1 °C), followed by a progressive tingling sensation, first mainly affecting the lower extremities and shortly moving to the upper extremities as well. He experienced a problematic and insecure gait, with a reduced sensation of soles while walking as well as decreased perception of grasped objects. At admission, the patient was COVID-19 negative (PCR test), normothermic (36.5 °C), and normotensive (125/85 mmHg) with 105 beats per minute. The patient was lucid, well oriented, without meningeal symptomatology, with isocoric pupils, correct optokinetics, and pupillary reflexes, without nystagmus. Facial expressions were symmetrical, without pathological axial reflexes, without signs of bulbar palsy, and the tactile perception of the face was symmetrically well preserved. Upper and lower extremities showed bilaterally symmetrical muscle tonus and muscle strength, and myotatic reflexes were symmetrically lowered, without signs of pyramidal tract and cerebellar involvement. Examination of tactile perception showed hypesthesia on all four extremities. Standing pose of the patient was independent but uncertain, with a positive Romberg’s sign. The gait was independent but only at short distances and uncertain. Phatic functions were normal; however, severe dysphonia and moderate dysphagia were detected. The patient did not report any similar symptoms in the past, nor has he suffered from any neurological condition prior to this. He was on chronic treatment with a combination of inhaler sympathomimetics and anticholinergics for chronic obstructive lung disease (GOLD A), and on a replacement therapy with levothyroxine (100 µg/day) due to thyroidectomy that the patients underwent in the past following subacute thyreoiditis with non-toxic diffuse goiter.
Laboratory workup at admission showed mildly elevated CRP (39.29 mg/l), D-dimer (0.92 mg/l FEU), and fibrinogen (7.54 g/l), with normal kidney and liver function. Examination of the cerebrospinal fluid showed protein-cytological dissociation with normal levels of glucose, lactate, chloride content, and increased levels of albumin (35.0 mg/l), total protein (0.54 g/l), and IgG (71.5 mg/l), without increased erythrocytes and leukocytes (< 2/ul).
Results of electromyography conducted on the second admission day indicated severe motoric and sensitive axonal demyelinating polyneuropathy of the lower extremities (with bilaterally markedly reduced CMAP (compound muscle action potential) and markedly prolonged H reflex). On the right leg, the segment of abductor hallucis-ankle showed a latency difference of 7.3 ms, the ankle-popliteal fossa segment had a latency difference of 22.3 ms, distance of 410 mm and conduction velocity of 18 m/s. On the left leg, the segment of abductor hallucis-ankle showed a latency difference of 6.4 ms, and ankle-popliteral fossa segment showed a latency difference of 18.8. ms, distance 420 mm and conduction velocity 22/ms. The activity in the neurogram of fibular nerve was absent. Left tibial nerve M-wave showed a latency of 8.2 ms and amplitude of 0.8 mV, and the H-reflex had a latency of 50.2 ms. Subsequent brain CT revealed leukoaraiosis without fresh and expansile lesions; without midline shift, the ventricular system was without dilation, and the subarachnoid space was normodense without signs of obstruction.
The diagnosis of Guillain-Barré syndrome was established as the most probable cause of patient’s deterioration based on the test results and clinical symptoms. The treatment with intravenous human immunoglobulins (IVIg) was started (30 g per day) on the third admission day, together with symptomatic treatment. Muscle weakness progressed, also affecting the respiratory muscles and leading to respiration insufficiency. The patient had to be analgosedated, intubated, and was started on mechanical ventilation. The inflammatory markers started to rise (CRP 75.22 mg/l). Chest X-ray showed diffuse ground-glass opacity over the right lung, and cultivation of bronchoalveolar lavage fluid detected multiresistant Pseudomonas aeruginosa (interpreted by clinicians as consistent with nosocomial infection in the setting of ventilated ICU patients). In spite of the treatment, the respiration insufficiency progressed to the development of acute respiratory distress syndrome, followed by hemodynamic instability and death 6 days after hospitalization and 10 days after the second dose of mRNA vaccine against COVID-19.
Subsequently, a full-body autopsy was conducted. The pleural cavity revealed firm adhesion between the visceral and parietal pleura on the right side. The lungs were bilaterally increased in size and weight (right 1260 g, left 950 g), of tough elastic consistency, with a large amount of turbid beige fluid coming out from the cut surface, with round areas of grey-pink merging consolidations in the lower lobes with multiple abscesses measuring 3 – 5 mm, filled with creamy beige-green substance. Histological examination indicated post-aspiration absceding bronchopneumonia as the immediate cause of death of the patient.
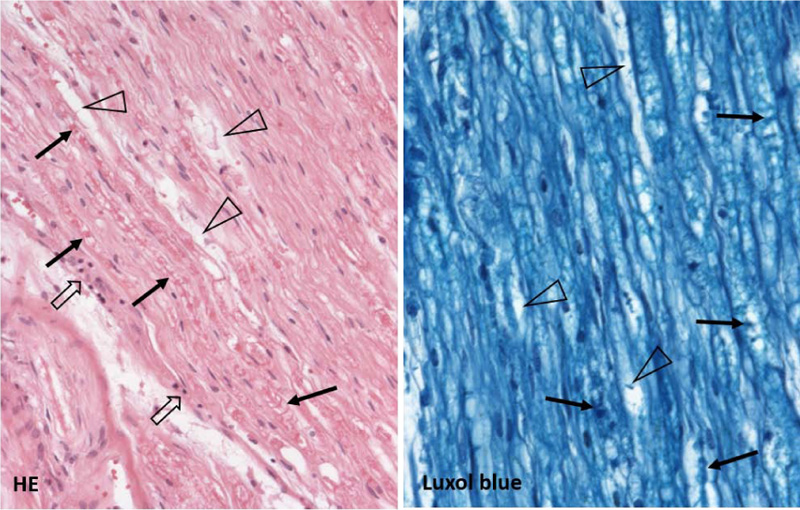
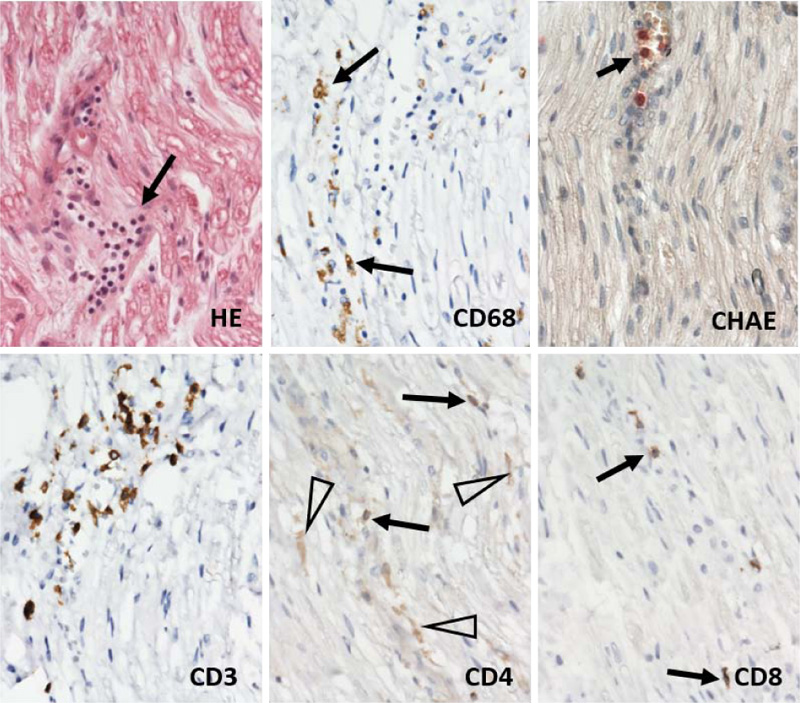
Gross and microscopic examination of the brain tissue and meninges did not reveal any pathological changes apart from slight edema. Histological examination of the spinal cord did not show any substantial changes in motoric neurons of the ventral roots nor in the captured spinal nerve roots. A thorough examination of the peripheral nerves of the lumbar plexus showed areas of focal demyelination (Fig. 1), prevalently perivascular infiltration by T-lymphocytes with a slight prevalence of T-cytotoxic over T-helper phenotype and the presence of numerous macrophages (Fig. 2). No significant penetration by polymorphonuclear leukocytes was observed. Described histological changes supported the clinical diagnosis of Guillain-Barré syndrome, an acute inflammatory demyelinating polyradiculoneuropathy (AIDP) subtype.
3. DISCUSSION
Guillain-Barré syndrome represents a group of potentially life-threatening neurological disorders developing typically after certain infections and less commonly following surgical procedures, selected drug intake as well as vaccinations [2]. GBS encloses several variants with variable clinical presentation, prognosis, as well as electrophysiological findings, including acute inflammatory demyelinating polyradiculoneuropathy (AIDP), acute motor axonal neuropathy (AMAN), acute motor and sensory axonal neuropathy (AMSAN), Miller-Fisher syndrome (MFS), and Pharyngeal-cervical-brachial variant. These subtypes present with different geographical distributions, with AIDP being the most common variant in North America and Europe [24].
GBS development is attributed to an autoimmune reaction aimed against constituents of the peripheral nervous system. The pathogenesis is not fully understood yet; however, a subset of GBS is considered to be driven by molecular mimicry, which is supported by the finding of serum antibodies that cross-react with gangliosides of peripheral nerves in a subset of susceptible GBS patients, mainly suffering from an axonal subtype of GBS resulting in nerve conduction failure and axonal degeneration [25, 26]. In the AIDP subtype, the targeting of the myelin sheets’ antigens leads to myelin degeneration by macrophages and T-lymphocytes [27, 28].
The symptoms of the AIDP variant include symmetrical and progressive neurological symptomatology, starting typically with paresthesias of the hands and feet and continuing as numbness, limb weakness, and areflexia, usually involving the lower extremities first [1, 25]. In other variants, the symptoms can affect different regions of the body, and vary from purely sensory to purely motor symptoms with their various combinations [25]. The progression of symptoms is usually rapid, with maximum muscle weakness within 5 – 12 days. Our patient reached the nadir on the 5th day after the initial symptoms occurred, the time when he needed mechanical ventilation. Generally, the duration of the disease plateau varies from 2 days to 6 months, followed by slow improvement, with the possibility of residual symptomatology in a subset of patients [2, 29].
The diagnosis of GBS relies on typical progressive clinical symptomatology and the elimination of other possible causes with similar neurological symptoms. The examination of cerebrospinal fluid is important; however, in a subset of patients, the parameters of CSF might show normal levels within the first week of symptom onset [29]. The diagnosis is further supported by nerve conduction studies; however, it might take up to two weeks for the electrophysiological abnormalities to be fully expressed [2, 29].
Histologic examination of the nerves is not a usual part of the diagnostic process; however, in disputed cases, it can aid the correct diagnosis. The typical histological presentation of the AIDP subtype includes patchily dispersed foci of demyelination, so it does not have to be necessarily captured during the sural nerve biopsy. There is typically irregular mononuclear infiltration, predominantly by T-lymphocytes and macrophages within the myelin sheaths. Histological findings in the peripheral nerve tissue of the presented case supported the clinical diagnosis of GBS, the AIDP subtype. AMAN and AMSAN subtypes show axonal degeneration, which is most prominent in the ventral spinal roots and paucity of lymphocytic infiltrate when compared to AIDP. There is typically central chromatolysis noted in the anterior horn cells of the spinal cord [30], which was not found in our autopsied patient.
Treatment of GBS that has proven to be effective consists of intravenous immunoglobulin (IVIg) or plasma exchange [2]. Early treatment together with continuous careful monitoring, prophylaxis of possible complications, timely rehabilitation, and psychological support represent important aspects of successful management of GBS patients.
Due to the involvement of respiratory muscles, patients can develop neuromuscular respiratory failure with the need of mechanical ventilation, followed in the majority of cases either by nosocomial or aspiration pneumonia, as in our case [31, 32]. Other complications include involvement of the autonomous nervous system, which can lead to instability of blood pressure and cardiac arrhythmias. The overall mortality of GBS patients is estimated to be around 3% [33, 34].
As of November 2021, there were 517 reports within the United States of America to VAERS (The Vaccine Adverse Event Reporting System) of Guillain-Barré syndrome possibly associated with COVID-19 vaccination by mRNA vaccine with 9 fatal cases. The reports represented less than 0.1% of all adverse event reports. The European database of suspected adverse drug reactions (EduraVigilance) recorded so far 1127 cases of GBS possibly associated with COVID-19 mRNA vaccine, with 19 fatal cases. GBS represented less than 0.2% of the reported adverse events associated with vaccination [35, 36].
At the time of writing this paper, the GBS cases reported in European Union and European Economic Area countries as a possible adverse effect after COVID-19 vaccination, regardless of the type of vaccination, represented 2605 cases, which stand for 0.0003% cases of all vaccine doses, which is less than expected in the general population regardless of vaccination status [36]. A case series study examining GBS admissions up to 28 days after the first dose of SARS-CoV-2 vaccine and COVID-19 infection in England showed an increased risk of GBS connected to AZD-1222 vaccine (incidence rate ratio 2.90); however, the risk of development of neurological complications, including GBS, is far greater after COVID-19 infection than vaccination [37].
With the progression of COVID-19 vaccination, reports of possible adverse events will rise. In cases of deaths associated with vaccinations, the reporting, as well as investigation of these, differs throughout countries. In Slovakia, all deaths reported as suspected after COVID-19 vaccination are investigated by full-body autopsy performed by professionals working for the Slovak Health Care Surveillance Authority in cooperation with the State Institute of Drug Control. As of November 2021, there were 1121 reports of possible serious side effects out of 5,117,618 doses of COVID-19 vaccinations. Out of these, there were 7 deaths interpreted to have a causal connection with vaccination, 4 deaths were categorized according to the WHO-UMC system as a possible adverse event following COVID-19 vaccination, and 3 deaths as a probable adverse event, including this described case and two cases leading to intracranial bleeding [38]. In any case, the reported cases of GBS are low, and even if some of the reported cases can be causally connected to vaccination, the overall risk of possible serious side effects remains very low.
CONCLUSION
The rollout of new vaccinations ensuing COVID-19 pandemic is naturally followed by a spike in adverse event reports, including a number of neurological conditions. A slightly increased risk of GBS development was associated with vector-based vaccines against COVID-19. In the case of mRNA vaccines, the rate of these so far does not seem to be higher than the incidence in the general population; however, the causality between some GBS cases and mRNA vaccination cannot be ruled out with certainty. Thus, doctors have to be vigilant about GBS symptomatology in the post-vaccination period to start timely management of these patients, and potentially reduce the fatal outcome of this diagnosis.
LIST OF ABBREVIATIONS
| AIDP | = Acute Inflammatory Demyelinating Polyradiculoneuropathy |
| AMAN | = Acute Motor Axonal Neuropathy |
| AMSAN | = Acute Motor And Sensory Axonal Neuropathy |
| CMAP | = Compound Muscle Action Potential |
| CRP | = C-Reactive Protein |
| CSF | = Cerebrospinal Fluid |
| CT | = Computed Tomography |
| GBS | = Guillian-Barré syndrome |
| GOLD | = Global Initiative for Chronic Obstructive Lung Disease |
| MFS | = Miller-Fisher Syndrome |
| PCR | = Polymerase Chain Reaction |
| UPC | = Uppsala Monitoring Center |
| VAERS | = The Vaccine Adverse Event Reporting System |
| WHO | = World Health Organization |
AUTHORS' CONTRIBUTIONS
PV and LP collected and interpreted the patient’s clinical data. KM, MV, PJ, and PB analyzed and interpreted the patient’s clinical data in association with autopsy data. KM, PB, and PV were major contributors to writing the article. MP provided data on adverse post-vaccination events. All authors read and approved the final manuscript.
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
Not applicable.
HUMAN AND ANIMAL RIGHTS
No animals/humans were used for studies that are the basis of this research.
CONSENT FOR PUBLICATION
The spouse of the deceased patient provided a written consent for publication of the case.
STANDARDS OF REPORTING
CARE guidelines have been followed in the preparation of this case report.
AVAILABILITY OF DATA AND MATERIALS
Not applicable.
FUNDING
This research was funded by Slovak Research and Development Agency (Grant number PP-COVID-20-051).
CONFLICTS OF INTEREST
The authors declare no conflicts of interest in preparing this case report.
ACKNOWLEDGEMENTS
Declared none.


