All published articles of this journal are available on ScienceDirect.
Effect of Sodium Valproate on Weight, Body Mass Index, Uric Acid, Vitamin D3, Blood Insulin, and Serum Lipid Profile in Children
Abstract
Introduction:
Due to the high prevalence of epilepsy and the use of sodium valproate as an antiepileptic drug by these patients, accurate recognition of its side effects and its effects on serum lipids profile, liver enzymes, uric acid level, and thyroid function tests, especially in cases that need long-term treatment seems essential. This study aimed to evaluate the effects of sodium valproate on weight, body mass index (BMI), vitamin D3, blood insulin, uric acid level, and serum lipids profile in children with newly diagnosed epilepsy.
Materials and Methods:
This prospective study was performed on 30 children between 3 and 8 years of age who suffered from newly diagnosed epilepsy and received sodium valproate as monotherapy. Data including demographic information (age, sex, height, weight, and waist and hip circumference of children), as well as clinical characteristics, such as liver enzymes (ALT, AST, ALK-P), serum lipids level (TG, TC, HDL-C, LDL-C), thyroid tests (TSH, T4), fasting blood sugar (FBS), uric acid level, 25 OH Vitamin D3 (Vit-D3), and blood insulin level of children before and six months after the consumption of sodium valproate, were examined.
Results:
The mean weight of children before and six months after the start of sodium valproate treatment was 18.54±2.99 and 21.13±3.93 (kg), respectively. This difference was statistically significant (P=0.005). Also, the mean weight Z-score of children before and after taking sodium valproate was -2.497 and -2.293, respectively, which was statistically significant too. In addition to weight gain, there was also a significant increase in the abdominal and hip circumference of children after taking valproate, whereas the increase in mean BMI before and after valproate administration was not statistically significant (P=0.114). However, mean weight gain, as well as the increase in the waist and hip circumference, had no relationship with gender (P> 0.05). Also, sodium valproate significantly increased the ALT level (P=0.046). Moreover, sodium valproate did not affect other liver function markers (AST), thyroid hormones (TSH, T4), fasting blood sugar (FBS), uric acid level, 25 OH Vit-D3, and the children's blood insulin levels (P> 0.05).
Conclusion:
According to the findings of this study, it can be concluded that sodium valproate is a good drug for children between 3 and 8 years of age, but it should be noted that taking this drug increases the chance of obesity in children. The main side effect of this drug is weight gain. Due to the significant increase in ALT enzyme, as observed in this study, it is recommended to check liver enzymes before, one, and six months after starting treatment as it can prevent the irreversible permanent side effects of this drug.
1. INTRODUCTION
Sodium valproate is a broad-spectrum drug that has been frequently prescribed as a first-line anticonvulsant since 1970 [1, 2]. Sodium valproate's multiple formulations show good absorption by binding to plasma proteins to a large extent. Sodium valproate has a half-life of around 8-19 hours and is quickly metabolized in the body [3]. Similar to other anticonvulsant drugs, sodium valproate comes with some side effects, including transient and harmless outcomes, such as weight gain, transient drowsiness, hair loss, hand-arm tremors at rest and activity, reversible thrombocytopenia, and moderate (about 3 times) increase in gamma-glutamyl transferase, as well as harmful complications, such as hepatotoxicity, encephalopathy, coagulation disorders, pancreatitis, and bone marrow suppression [4]. Sodium valproate interacts with drugs, such as phenobarbital, phenytoin (PHT), carbamazepine, lamotrigine, felbamate, rifampin, ethosuximide, and primidone. It generally confers suitable therapeutic properties, and whether it is used or prohibited or its modification due to negative side effects should be decided individually for each patient [3]. Obesity or an increase in adipose tissue is defined using the body mass index (BMI), where the body weight (kg) is divided by the square of the body height (m). In a child over 2, obesity is defined as a BMI at or above the 95th percentile, and a BMI at the 85th to 95th percentile means that the person is overweight [5, 6]. Weight gain is a well-known outcome of treatment with sodium valproate that is found in 40% of children [7] and is also the most prevalent reason for terminating the treatment of patients with sodium valproate [8]. Recently, 38% of patients treated with sodium valproate experienced weight gain of more than 10% of their body weight, which was 8% higher than patients treated with lamotrigine [9]. In addition, weight gain following the consumption of sodium valproate seems to be associated with increased appetite and is not metabolic [10]. Despite knowing the correlation between weight gain and treatment with sodium valproate, the pathogenic mechanisms of this adverse effect are still undiscovered. Numerous studies have reported that antiepileptic drugs, such as phenytoin, phenobarbital, and carbamazepine, increase the serum concentration of HDL-C, while some others do not confirm these effects. Furthermore, some researchers have reported that valproic acid and other novel antiepileptic drugs, such as lamotrigine and levetiracetam, do not affect serum lipid profiles [11]. Some recent studies have further shown that treatment with sodium valproate is associated with increased serum concentration of insulin and variations in the concentration of triglyceride (TG) and high-density lipoprotein (HDL-C) cholesterol. Verotti et al. showed that the prevalence of metabolic syndrome in children and adolescents who gained weight following the treatment with sodium valproate was similar to that of healthy overweight people [12, 13]. However, another study reported that obese patients with epilepsy treated with sodium valproate were at higher risk for metabolic syndrome than controls [13]. Serum concentrations of specific lipids and lipoproteins in young adults are serious risk factors for the development of cardiovascular disease during life. Several data showed that increased total cholesterol, elevated triglyceride (TG) and LDL-C, and decreased HDL-C cause cardiovascular disease. Therefore, the assessment of variations in serum lipid profile levels following the consumption of anticonvulsants may help select the safest drug to prevent cardiovascular complications [14]. Knowing that the complications of sodium valproate may be higher in children than adults and due to the importance of identifying these outcomes to deal with them, we conducted this prospective study to investigate the effect of sodium valproate on weight, BMI, serum lipids profile, the serum concentration of insulin, uric acid, and thyroid tests in children treated at the Hazrate Rasool Akram Hospital in Tehran, Iran.
2. MATERIALS AND METHODS
2.1. Inclusion Criteria
All children between 3 and 8 years of age with newly diagnosed epilepsy who were candidates for sodium valproate and had no metabolic disease or underlying chromosomal condition, obesity, FTT, or congenital anomalies were included in the study.
2.2. Exclusion Criteria
Exclusion criteria included underlying conditions, such as chronic hepatic, heart, renal, and metabolic diseases, diabetes, chromosomal disease, obesity, FTT, congenital anomalies, progressive neurological disease, gastrointestinal diseases, coagulation disorders, and developmental delay.
2.3. Methods
It was a prospective study in which, following coordination with the ethics department of Iran university of medical sciences, children between 3 and 8 years of age, who were admitted to the pediatric ward of the Rasool Akram Hospital in Tehran from 2018 to 2019 due to newly diagnosed epilepsy and were candidates for sodium valproate treatment, were examined. All patients were from the Iranian population at different socioeconomic levels. In all patients, sodium valproate was started at a dose of 10 mg/Kg and increased to 20 mg/Kg within two weeks and the serum level of this drug was checked after one month of consumption which was 50-100mcg/ml. Demographic characteristics (age and gender), anthropometric features (height, weight, and circumference of waist and hip), as well as clinical characteristics, such as liver enzymes (ALT, AST, ALK-P), lipids profile (TG, TC, HDL-C, LDL.C), thyroid tests (TSH, T4), fasting blood sugar (FBS), uric acid, 25 OH vitamin D3 (Vit-D3), and blood insulin level, were evaluated in two turns, before the beginning of sodium valproate and after 6 months of treatment, in each child. All of the above factors in 30 children aged 3 to 8 years (4.9 ± 1.64 on average) who were treated with sodium valproate due to newly diagnosed epilepsy were examined before and after 6 months of treatment.
2.4. Data Analysis
Data were analyzed in IBM, SPSS, Statistics v22.0 using descriptive statistics, frequency tables, and scatter indices, such as mean and standard deviation (SD). The relationship between variables was analyzed by Chi-squared (χ2) and Fisher's exact tests. The t-test was used to compare the mean of two normal, independent quantitative variables, and the Kolmogorov-Smirnov test was applied to compare abnormal variables. The Mann-Whitney nonparametric test was used to compare the mean in two independent groups and the Kruskal-Wallis nonparametric test was used to compare the mean in more than three independent groups. The statistical tests, i.e., the Wilcoxon test, the Pearson correlation coefficient test, and the Spearman correlation coefficient test, were used for data analysis. In all cases, a significance level of 0.05 was considered.
3. RESULTS
In this study, demographic information (such as age and gender), anthropometric features (e.g., weight, height, BMI, and the circumference of waist and hip), and also paraclinical characteristics, such as hepatic enzymes (ALT, AST, ALK- P), lipids profile (TG, TC, HDL-C, LDL.C), thyroid tests (TSH, T4), fasting blood sugar (FBS), the concentration of uric acid, 25 OH vitamin D3 (Vit-D3), and the level of blood insulin in 30 children aged 3 to 8 years (4.9 ± 1.64 on average) who were treated with sodium valproate due to newly diagnosed epilepsy were evaluated before and after 6 months of treatment. Out of 30 children, 12 children (40%) were female and 18 (60%) were males, with the mean age of respectively 5 ± 1.41 and 4.8 ± 1.91. There was no significant difference between boys and girls (P = 0.88) (Fig. 1).
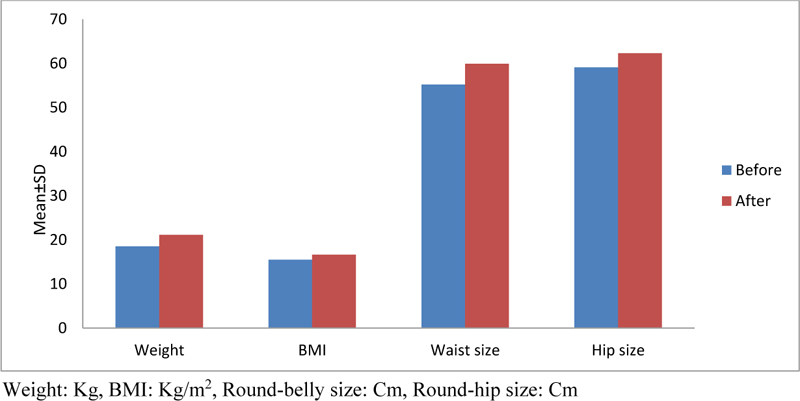
Evaluation of weight (Kg and Z score), BMI (kg/m2), circumference of the abdomen, and hip (cm) in children before and 6 months after consuming sodium valproate revealed that the mean weight of children before and after taking valproate was 18.54±2.99 and 21.13±3.93, respectively, which was statistically significant (P = 0.005). Also, the mean weight Z-score of children before and after taking sodium valproate was -2.497 and -2.293, respectively, which was statistically significant too. The mean BMI of children before and after taking valproate was 15.48±1.97 and 16.66±2.72, respectively, which indicates no significant difference (P = 0.114). The mean size of the abdomen in children was 55.2±15.29 and 59.9±15.29, respectively, before and after taking sodium valproate, which is statistically significant (P = 0.005). The mean hip circumference in children after consuming sodium valproate was increased significantly from 59.1±6.10 to 62.3±7.63 (P = 0.008). Table 1 indicates the mean values of weight (Kg), BMI, and circumference of the abdomen and hips in children before and after taking sodium valproate.
Mean values of weight, body mass index, and circumference of the abdomen and hips of children before and after taking sodium valproate were compared individually among girls and boys. Before starting sodium valproate, the mean weight, body mass index, and abdominal and hip circumference of girls and boys were 18.4±1.12 and 18.6±3.91, 14.9±1.83 and 15.87±2.13, 49.25±3.27 and 59.16±19.16, 57.7±2.10 and 60±7.87, respectively. Then, following the consumption of sodium valproate, the mean weight, body mass index, and abdominal and hip circumference of girls and boys were 20.95±2.19 and 21.25±4.99, 16.04±1.32 and 17.08±3.42, 55±5.29 and 63.16±19.16, 61±3.36 and 63.16±9.78, respectively. These showed no significant difference between girls and boys (P> 0.05). Table 2 presents the mean values of weight, body mass index, and circumference of children's abdomen and hips before and after taking sodium valproate by gender.
| Variables | After using Sodium valproate |
Before Using Sodium valproate |
P-Value | ||
|---|---|---|---|---|---|
| Range | Mean ± SD | Range | Mean ± SD | ||
| Weight | 15-27/7 | 21/13 ± 3/93 | 13/6-24 | 18/54 ± 2/99 | 0/005* |
| BMI | 15-24 | 16/66 ± 2/72 | 13/1-18/5 | 15/48 ± 1/97 | 0/114* |
| Round-bellied size | 48-99 | 59/9 ± 15/29 | 46-97 | 55/2 ± 15/29 | 0/005* |
| Round-hip size | 53-78 | 62/3 ± 7/63 | 51-70 | 59/1 ± 6/10 | 0/008* |
| Variable | After using Sodium valproate | *P-value | Before using Sodium valproate | *P-Value | ||
|---|---|---|---|---|---|---|
| Girls | Boys | Girls | Boys | |||
| Weight | 20/95 ±2/19 | 21/25 ± 4/99 | 0/915 | 18/4 ± 1/12 | 18/6 ± 3/91 | 0/831 |
| BMI | 16/04 ± 1/32 | 17/08 ± 3/42 | 0/522 | 14/9 ± 1/83 | 15/87 ± 2/13 | 0/670 |
| Round bellied size | 55 ± 5/29 | 63/16 ± 19/16 | 0/336 | 49/25 ± 3/27 | 59/16 ± 19/16 | 0/669 |
| Round-Hip size | 61 ± 3/36 | 63/16 ± 9/78 | 0/593 | 57/7 ± 2/10 | 60 ± 7/87 | 1 |
Paraclinical features, such as hepatic enzymes (ALT, AST, ALK-P), lipids profile (TG, TC, HDL-C, LDL.C), thyroid tests (TSH, T4), fasting blood sugar (FBS), the concentration of uric acid, 25 OH Vitamin D3, and blood insulin level, were assessed before and after consuming sodium valproate. Table 3 presents the mean values of pediatric paraclinical variables before and after taking sodium valproate. The mean value of hepatic enzyme alanine aminotransferase (ALT) has been increased meaningfully after consuming sodium valproate (P = 0.04), i.e., from 15.6±5.69 to 20.3±7.24. Other hepatic enzymes (AST, ALK-P), level of blood lipids (TG, TC, HDL-C, LDL-C), thyroid tests (TSH, T4), fasting blood sugar (FBS), the concentration of uric acid, 25 OH Vitamin D3 (Vit-D3), and the level of blood insulin in children before and after consuming sodium valproate did not alter significantly (P> 0.05).
Paraclinical features, such as hepatic enzymes (ALT, AST, ALK-P), lipids profile (TG, TC, HDL-C, LDL.C), thyroid tests (TSH, T4), fasting blood sugar (FBS), the concentration of uric acid, 25 OH Vitamin D3, and the content of blood insulin before and after consuming sodium valproate were measured individually in boys and girls. Table 4 presents the mean values of pediatric paraclinical variables before and after taking sodium valproate in girls and boys. Based on the results obtained, no significant difference was observed between the means of paraclinical data in girls and boys, both before and after taking sodium valproate (P> 0.05).
The Spearman test was used to examine the correlation between the variables. The results for meaningful variables that are related to each other are given in Table 5. According to the results, an increase in the size of children's hips is related to an increase in the size of their waist, an increase in the content of TSH is related to an increase in the content of AST, an increase in the content of FBS is related to an increase in the concentration of HDL, an increase in the content of TG is related to an increase in the concentration of vitamin D3, and an increase in the concentration of LDL is related to an increase in the concentration of cholesterol. However, there is a converse relationship between the two variables of alkaline phosphatase (ALK-P) and HDL (R = -0.695, P = 0.026), so an increase in the concentration of one variable is related to a decrease in the concentration of another variable and vice versa.
| Variants | After using Sodium valproate | Before using Sodium valproate | P-Value | ||
|---|---|---|---|---|---|
| Range | Mean ± SD | Range | Mean ± SD | ||
| ALT | 12-35 | 20/3 ± 7/24 | 10-28 | 15/6 ± 5/69 | 0/046* |
| AST | 18-40 | 30/4 ± 7/27 | 20-40 | 30/9 ± 7/47 | 0/838 |
| ALK-P | 395-677 | 524/8 ± 94/91 | 387-682 | 530/1 ± 100/1 | 0/959 |
| TC | 107-209 | 140/9 ± 29/24 | 110-172 | 139/81 ± 21 | 0/919 |
| HDL | 33-55 | 39/8 ± 6/17 | 32-51 | 40/2 ± 6/47 | 0/858 |
| LDL | 59-130 | 82/8 ± 19/88 | 40-93 | 73/3 ± 16/6 | 0/333 |
| TG | 30-262 | 93/1 ± 66/53 | 38-239 | 105/2 ± 60/13 | 0/386 |
| TSH | 1/5-7/9 | 3/25 ± 1/99 | 0/26-3/5 | 1/96 ± 0/96 | 0/103 |
| FT4 | 6-11/2 | 8/49 ± 1/86 | 7/1-9/5 | 8/1 ± 0/71 | 0/507 |
| FBS | 74-112 | 90/7 ± 11/3 | 41-147 | 92 ± 29/9 | 0/575 |
| Uric-Acid | 2/5-5/2 | 4/16 ± 0/75 | 1/8-6/3 | 4/5 ± 1/2 | 0/185 |
| Vit-D3 | 10/7-49 | 22/43 ± 10/27 | 9/5-35 | 22/9 ± 8/07 | 0/799 |
| Insulin | 1/4-23/7 | 6/37 ± 6/64 | 2-28/6 | 8/53 ± 8/41 | 0/508 |
| Variants | After using Sodium valproate | *P-Value | using Sodium valproate | *P-Value | ||
|---|---|---|---|---|---|---|
| Girls | Boys | Girls | Boys | |||
| ALT | 17/5 ± 5/91 | 22/16 ± 7/93 | 0/195 | 12/5 ± 3 | 17/66 ± 6/34 | 0/336 |
| AST | 28/2 ± 6/39 | 31/83 ± 8/03 | 0/454 | 34 ± 5/4 | 28/8 ±8/35 | 0/454 |
| ALK-P | 487 ± 92/78 | 550 ± 95/62 | 0/336 | 530/5 ± 119 | 529/8 ± 97/6 | 0/522 |
| TC | 128/5 ± 14/27 | 149/16 ± 34/81 | 0/394 | 131/5 ± 29/01 | 144 ± 16/63 | 0/286 |
| HDL | 38/25 ± 4/78 | 40/83 ± 7/19 | 0/240 | 44 ± 7/78 | 37/6 ± 4/45 | 0/666 |
| LDL | 74/5 ± 8/81 | 88/33 ± 23/93 | 0/670 | 70 ± 20/31 | 75/5 ± 15/3 | 0/286 |
| TG | 82 ± 25/97 | 100/5 ± 86/01 | 0/522 | 90/5 ± 60/22 | 115 ± 63/6 | 0/670 |
| TSH | 3/42 ± 2/99 | 3/13 ± 1/30 | 0/055 | 1/26 ± 0/67 | 2/42 ± 0/85 | 0/392 |
| FT4 | 7/65 ± 1/62 | 9/05 ± 1/92 | 0/281 | 8/4 ± 0/82 | 7/86 ± 0/61 | 0/240 |
| FBS | 87/25 ± 10/62 | 93 ± 12/09 | 0/517 | 92 ± 43/3 | 92 ± 21/9 | 0/453 |
| Uric-Acid | 3/8 ± 0/92 | 4/4 ± 0/57 | 0/831 | 4/12 ± 1/59 | 4/73 ± 0/94 | 0/391 |
| Vit-D3 | 27/17 ± 15/1 | 19/2 ± 4/81 | 0/914 | 23/3 ± 11/04 | 22/6 ± 6/62 | 0/522 |
| Insulin | 5/05 ± 3/58 | 7/25 ± 8/33 | 1 | 7/47 ± 6/68 | 9/23 ± 9/94 | 1 |
| Variants | Correlation coefficients (r) | P-Value |
|---|---|---|
| waist size, hip size | 0/812 | 0/004 |
| TSH, AST | 0/75 | 0/012 |
| ALK-P, HDL | - 0/695 | 0/026 |
| FBS, HDL | 0/667 | 0/035 |
| TG, Vit-D3 | 0/709 | 0/022 |
| LDL, Chol | 0/979 | <0/001 |
4. DISCUSSION
This study was carried out in 2018 on 30 children aged between 3 to 8 years who were admitted to the pediatric ward of the Rasool Akram Hospital with newly diagnosed epilepsy and were treated with sodium valproate. In this study, factors, including gender, age, weight, height, and size of waist and hip, as well as hepatic enzymes, and the results of laboratory testing of lipids, uric acid, thyroid hormones, vitamin D3, and FBS were recorded before and 6 months after consuming sodium valproate for each patient. The results showed a weight gain following the consumption of sodium valproate in all children (100%). The mean weight of children before and 6 months after taking sodium valproate was 18.54±2.99 and 21.13±3.93, respectively, which was statistically significant (P = 0.005). Also, the mean weight Z-score of children before and after taking sodium valproate was -2.497 and -2.293, respectively, which was statistically significant too. A significant increase in the abdomen and hip circumferences of children, besides weight gain, was observed after taking sodium valproate, while the increase in the mean BMI of children before and after consuming valproate was not statistically meaningful (P = 0.114). There was no correlation between the increase in mean weight, as well as the size of waist and hip circumference of children, and gender (P> 0.05). The results of this study are consistent with the results of several similar studies conducted previously. According to previous studies, weight gain is a serious side effect of treatment with sodium valproate, which occurs in almost half of patients, both children and adults, and is associated with major metabolic and endocrine disorders [8, 15-17]. Nasr Esfahani et al. [15] reported 53.1% weight gain in children, and Yaghini et al. [16] reported 51.6% weight gain in children treated with sodium valproate. Egger et al. [18] studied 100 epileptic children treated with sodium valproate in 1981 and found that the most frequent outcome of sodium valproate is weight gain, which occurred in 44 patients (44%). Ghofrani et al. [19] reported 40% weight gain in children following the consumption of sodium valproate. A study conducted by Novak et al. [20] on 55 children (30 females and 25 males) showed that weight gain and body mass index were directly related to the patient's weight and initial BMI at the start of treatment but they were not related to gender, age of the patient at the start of treatment, and the duration of medication.
According to some previous studies, treatment with sodium valproate is also associated with increased serum concentration of insulin [17, 21] and altered triglyceride (TG), high-density lipoprotein (HDL-C), low-density lipoprotein (LDL-C), cholesterol [14, 22], as well as an influence on hepatic tests (ALT, AST, ALK-P) [22, 23]. Therefore, it is vital to monitor the serum concentration of lipids and liver tests to prevent liver complications and adjust the dose of the drug during treatment. In our study, the level of serum insulin and serum lipids, total cholesterol, TG, HDL, and LDL cholesterol, as well as liver enzymes ALT, AST and ALK-P were compared after and before taking sodium valproate. The results showed that sodium valproate consumption only causes an increase in ALT enzyme so the initial amount of this enzyme in children was 15.6±5.6 and increased by 20.3±7.2 after taking sodium valproate, which was statistically significant (P = 0.046). However, sodium valproate does not affect other liver enzymes, such as AST and ALK-P, as well as serum lipid and serum insulin status (P> 0.05). According to our findings, there was no significant difference between boys and girls in increasing ALT, and an increase was observed in both boys and girls regardless of gender (P> 0.05). Anticonvulsant drugs have been shown to alter the serum concentration of lipid by acting on liver microsomal enzymes [24]. In a study on 38 children (20 females and 18 males) aged between 8.6±3.9 on average, serum lipids and liver function test results at the start and in the second and sixth months of treatment with valproic acid were examined and the findings showed that sodium valproate did not affect them [22]. The authors concluded that by comparing the results of this study with previous studies, there is clear heterogeneity in the results, but in general, it is not recommended to evaluate the concentration of serum lipids routinely in all of the people treated with anticonvulsants, such as sodium valproate. Due to the significant increase in ALT enzyme seen in this study, it is recommended that liver tests should be conducted before and one and six months after starting treatment as they can prevent the irreversible permanent side effects of the drug.
Other side effects of anticonvulsants include their effect on the function of the endocrine glands (such as the thyroid). Many drugs can alter the content of T3, T4, and TSH, including anticonvulsants, such as phenobarbital, carbamazepine, and sodium valproate. These drugs induce the hepatic microsomal enzyme system and affect the metabolism of thyroid hormones [25]. Several studies have reported various effects of antiepileptic drugs on the function of thyroid hormone [26, 27]. In a study by Thomas et al. [28], conducted in India, thyroid hormone levels in 43 epileptic patients were compared with controls and it was reported that the concentration of T4, T3, and FT3 was significantly lower and the concentration of TSH and FT4 was significantly higher than the control group. Also, it showed that the concentration of thyroid hormone was low in patients with epilepsy treated with carbamazepine or diphenylhydantoin, with lower prevalence among patients treated with sodium valproate and phenobarbital. Therefore, anticonvulsant drugs can increase the peripheral catabolism of FT3, T3, T4 by inductive enzymes. Several other studies have examined thyroid function in epileptic patients and suggested that no change is directly related to epilepsy itself, but some variations in thyroid function tests may be related to some anticonvulsant drugs. However, laboratory variations may not be significant. In this study, serum levels of TSH and free T4 in all patients before and after treatment were examined to evaluate the effects of sodium valproate on thyroid hormones. The mean serum levels of TSH and free T4 were increased after treatment, but this increase was not statistically significant (P>0.05). According to the results of this study, in general, sodium valproate does not affect the level of thyroid hormones in children.
A study conducted by Attilakos et al. revealed that sodium valproate has no effect on serum uric acid levels in children [29]. There have not been many studies on the effect of sodium valproate on serum uric acid levels in children. Based on our knowledge, this study is the second one, indicating that sodium valproate has no effect on serum uric acid levels in children.
In our study, serum lipids, liver enzymes, thyroid hormones, fasting blood sugar (FBS), uric acid level, 25 OH vitamin D3 (Vit-D3), and blood insulin levels were measured and analyzed before and 6 months after treatment with sodium valproate to evaluate the effects or side effects of this drug in children. However, no significant change was observed in all cases (P> 0.05) except for the ALT enzyme. Due to the normal serum level of insulin, uric acid, and lipids, it seems that the role of sodium valproate in children's weight gain is more a result of increased appetite than metabolic and hormonal changes.
CONCLUSION
Weight gain following the consumption of sodium valproate was observed in all children in this study. In addition to weight gain, a significant increase was observed in the size of the abdominal and hips circumference of children after taking this drug. Furthermore, treatment with sodium valproate causes a significant increase in the ALT enzyme. Due to the normal serum level of insulin, uric acid, and lipids, it seems that the role of sodium valproate in children's weight gain is more a result of increased appetite than metabolic and hormonal changes. Sodium valproate is a suitable drug for children aged between 3 and 8 years, but in general, the consumption of this drug increases the chances of obesity in children with overweight as one of the main side effects. Based on our findings, it is not recommended to evaluate the serum concentration of lipids, thyroid hormones, FBS, serum insulin, and serum uric acid in patients treated with sodium valproate. Due to the significant increase in ALT enzyme reported in this study, it is recommended that liver tests should be examined before and one and six months after starting treatment as they can prevent the irreversible permanent side effects of the drug.
AUTHORS' CONTRIBUTIONS
VM, SF, and MP designed and conducted the study. BN and TA analyzed and interpreted the patient’s data and participated in the follow-up duration of the patients. RA contributed to the writing of the manuscript. All of the authors read and approved the final manuscript.
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
The thesis was approved by the medical ethics committee of the Iran University of Medical Sciences, Iran (Approval no. ID of IR.IUMS.FMD.REC.1397.203).
HUMAN AND ANIMAL RIGHTS
No animals were used in the studies that are the basis of this research. All the humans used were in accordance with the ethical standards of the committee responsible for human experimentation (institutional and national), and with the Helsinki Declaration of 1975, as revised in 2013.
CONSENT FOR PUBLICATION
The consent for publication of personal detailed data was obtained from parents.
STANDARDS OF REPORTING
STROBE guidelines and methodologies were followed in this study.
AVAILABILITY OF DATA AND MATERIALS
The data supporting the findings of the article are available at the website: https://www.dropbox.com/home/4.%20Sodium %20Valproate, reference number 4.
FUNDING
None.
CONFLICT OF INTEREST
The authors declare no conflict of interest, financial or otherwise.
ACKNOWLEDGEMENTS
The present research article has been extracted from the “Thesis” of Dr. Nafise Sadat Borghei (approved by the medical ethics committee). The authors would like to thank the vice chancellor for research at the Iran University of Medical Sciences.


