All published articles of this journal are available on ScienceDirect.
An Overview of Studies Demonstrating that ex vivo Neuronal Networks Display Multiple Complex Behaviors: Emergent Properties of Nearest-Neighbor Interactions of Excitatory and Inhibitory Neurons.
Abstract
The responsiveness of the human nervous system ranges from the basic sensory interpretation and motor regulation to so-called higher-order functions such as emotion and consciousness. Aspects of higher-order functions are displayed by other mammals and birds. In efforts to understand how neuronal interaction can generate such a diverse functionality, murine embryonic cortical neurons were cultured on Petri dishes containing multi-electrode arrays that allowed recording and stimulation of neuronal activity. Despite the lack of major architectural features that govern nervous system development in situ, this overview of multiple studies demonstrated that these 2-dimensional ex vivo neuronal networks nevertheless recapitulate multiple key aspects of nervous system development and activity in situ, including density-dependent, the spontaneous establishment of a functional network that displayed complex signaling patterns, and responsiveness to environmental stimulation including generation of appropriate motor output and long-term potentiation. These findings underscore that the basic interplay of excitatory and inhibitory neuronal activity underlies all aspects of nervous system functionality. This reductionist system may be useful for further examination of neuronal function under developmental, homeostatic, and neurodegenerative conditions.
1. INTRODUCTION
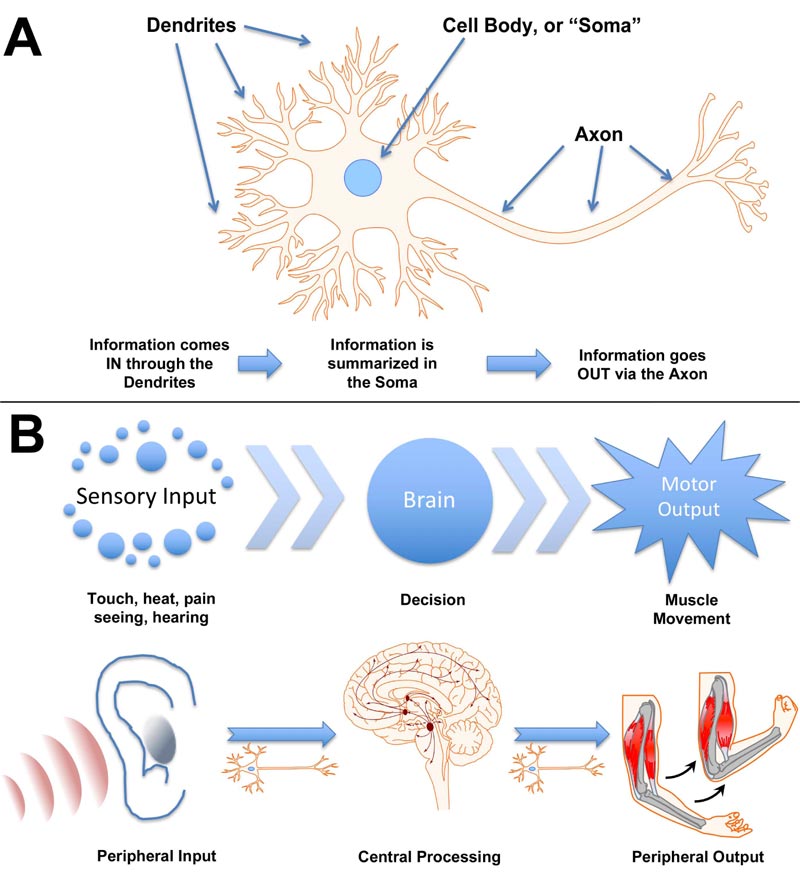
Integration of complex environmental information and generation of appropriate responses are mediated by neurons [1]. Dendrites collect sensory input, which is integrated at the cell body and summarized as a “fire/no fire” action via the axon and associated synapse (Fig. 1A). The simplest response, such as that of a hydra, consists of sensory input motor output, mediated by simple neuronal connections of primitive ganglia [2]. Notably, however, evidence has been presented that multifunctional neurons of the hydra may have functioned essentially as neuronal stem cells that yielded sensory, motor, and interneurons as well as neurosecretory cells observed in more complex nervous systems [3]. It has been suggested that the simple evolutionary development of “integration centers” on opposite ends of a simple nerve net ultimately generated the bilaterian nerve cord and brain of complex animals [4]. A complex nervous system, with central and peripheral systems, recapitulates the function of individual neurons: sensory neurons collect information from the environment, transmit this information to inter-neurons where it is integrated, and a response is mediated by motor neuronal output (Fig. 1B). To consider the integration of nervous system function, the sensory neurons as a collective can be considered as analogous to dendrites of a single neuron, the interneurons analogous to the soma of that neuron, and the motor neurons as analogous to the axonal output of that neuron.
While we tend to regard ourselves as intellectual creatures, it is important to consider that our nervous system, including extremely well-developed cortical regions, shares overall homology with that of other animals [5] and remains critically dependent upon sensory input. Our interaction with our environment, including learning, logic, and formation of memories, is still filtered through the limbic system. It has been suggested that primitive emotions may underly the evolutionary development of consciousness and that aspects of consciousness are experienced by animals as well as humans [6-9]. “Neural Correlates of Consciousness” are considered to be the physical cortical regions that generate aspects of consciousness [6, 10]. Moreover, consciousness can be defined as relatively simple responses to the environment, adaptation, and establishment of long-term memory (considered as “primary consciousness”) versus self-awareness and complex language (“higher-order consciousness”) [11]. Higher-order consciousness may be restricted to humans and/or primates [11], and primary consciousness is widely present among animals [12, 13]. This line of reasoning is consistent with the wide-spread distribution of general neuronal features among animals [14]. Consciousness, especially higher-order consciousness, may be considered as the ultimate emergent property of the nervous system, and its emergence, like any neuronal response, can be distilled down to the integration of synaptic transmission.
The reductionist approach of monitoring neuronal interactions in culture provides a unique analysis of the nervous system development and function of the nervous system. Resultant ex vivo neuronal networks display multiple aspects of primary consciousness, including complex signaling regulated by the extent of synaptic connections, responsiveness to the environment including the appropriate direction of motor activity, and long-term potentiation (which is the cellular basis for learning and memory). We will examine these in detail in the following sections.
We discuss these topics both in the context of a review/overview as well as to serve as potential starting points for culturing of neurons on MEAs. Illustrations and text are drawn largely from our previous publications, with appropriate references in the text and figure legends. Readers are encouraged to review these prior publications for more detail.
2. GENERAL METHODS
Establishing ex vivo neuronal networks is accomplished via the basic methods for culturing neurons, in either dissociated or slice versions, with the only difference being that electrodes are embedded in the culture chamber base. These methodologies are presented in detail in the primary articles e.g., [15-26]. Particular methodologies are summarized in each of the following sections.
Regarding eligibility criteria for studies reviewed herein, in accordance with Cochrane Community guidelines (https://community.cochrane.org/mecir-manual/standards-conduct-new-cochrane-intervention-reviews-c1-c75/developing-protocol-review-c1-c23/setting-eligibility-criteria-including-studies-review-c5-c13), we made every attempt to include all relevant references, resulting in 81 total references, and did not deliberately exclude any MEA studies.
3. DEVELOPMENT OF SYNAPTICACTIVITY: MONITORING BY MULTI-ELECTRODE ARRAYS AND COMPUTER INTERFACE
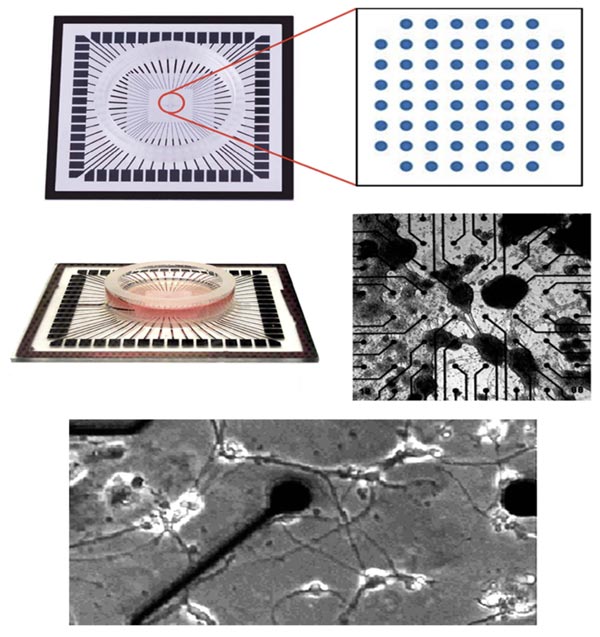
Microelectrode Arrays (MEA) consist of a series of electrodes, typically embedded within a petri dish, that provide the ability to record neuronal activity. MEAs have been used in many studies to stimulate individual and/or multiple neurons and record their responses [15-17]. MEAs allow direct delivery of multiple spatiotemporal patterns of stimulation, in a manner not achievable in situ [18-20].
Dissociated cultures of cortical cells grown on multi-electrode Arrays (MEAs) have been used in a wide variety of studies of network physiology. These studies described fundamental properties of neuronal network activity patterns, plasticity, models of learning and epilepsy, and pharmacological testing [20-26].
Various MEAs are commercially available (Fig. 2). They can be used to analyze signaling during the establishment of networks as well as during network manipulation via stimulation or treatment with various agents [18-20, 22, 23].
4. SPONTANEOUS DEVELOPMENT OF COMPLEX SIGNALING IN NEURONS
In dense cultures, neurons are often aggregated (e.g., Fig 3A). Minor baseline fluctuations observed in the absence of Bone fide signals but in the presence of neurons were readily distinguished from the minor baseline fluctuations observed in their absence (Fig. 3B). Cessation and restoration of this activity following addition and withdrawal of synaptic blockers such as tetrodotoxin confirmed this activity in the presence of neurons to be synaptic (Fig. 3B). Spontaneous signals, which occurred as individual spikes at irregular intervals, were observed within one week following culturing. By contrast, at 20 days after plating, streams of continuous spikes and “bursts” (defined as clusters of signals in which deviated from baseline for at least 0.7 sec) [27] were regularly appeared (Fig. 3C). These streams predominated for the following15 days. Bursts persisted throughout the time during which cultures were monitored [27]. Over time, the number of spikes decreased while bursts persisted, which eventually resulted in an overall prevalence of bursts within 1 month after plating [27]. Astroglia were routinely allowed to proliferate and typically obscured the neuronal network over time (Fig 3E and F). Long-term cultures (≥1 month in culture), operationally defined as “mature” cultures, displayed spontaneous signals that radiate across substantial regions, recorded by the simultaneous appearance at multiple electrodes, yet absent from adjacent electrodes (Fig. 3G and H) [28]. These findings indicate functional synaptic connectivity of neurons within cultures, resulting in a neuronal network. Networks remained functional for months [29]. We did not examine whether or not there was any selectivity among neurons for synaptogenesis. However, networks were generated by random deposition of neurons into a 2-dimensional surface, lacking the architectural restrictions and organizing aspects observed in situ [30]. Therefore, synaptogenesis in these ex vivo neuronal networks can be considered as occurring on a “nearest-neighbor” basis.
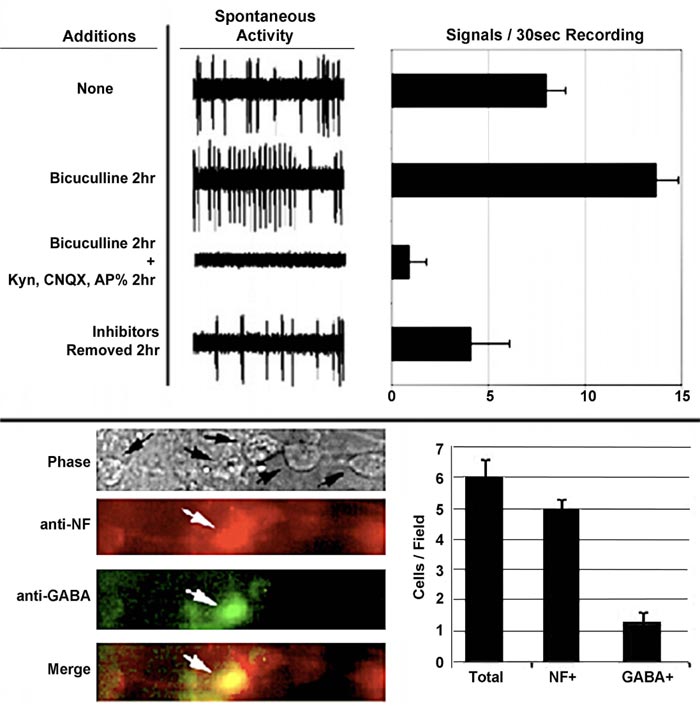
The central nervous system contains excitatory and inhibitory neurons, and its functionality is dependent upon their interaction. We examined whether or not the interaction of excitatory and inhibitory neurons was responsible for the development of mature spontaneous signaling patterns. To accomplish this, bicuculline, an antagonist of receptors for the inhibitory neurotransmitter gamma-aminobutyric acid (GABA), for 2 h [31] was added to neuronal networks. The addition of bicuculline increased the number and amplitude of signals. This indicated that inhibitory neurons were responsible for restricting overall spontaneous activity Fig. (4) [32, 33]. Notably, the overall loss of signals following the addition of this excitatory antagonist confirms that signals, including the increase following the addition of bicuculline, are derived from the excitatory neuronal synaptic activity.
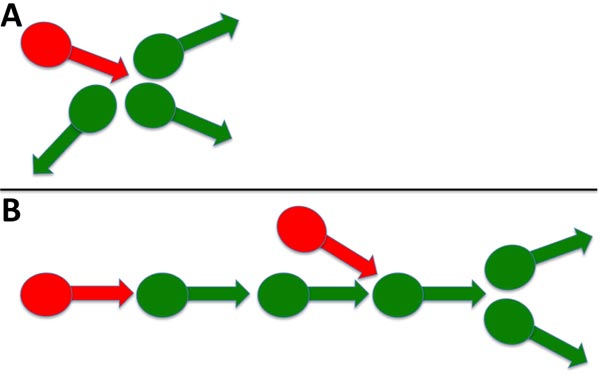
The emergence of complex signaling (i.e., including bursts) was density-dependent. Sparse neuronal cultures did not convert to bursts but instead displayed continuous spikes, while dense cultures displayed predominantly bursts over time (Fig. 5) [32]. Similar results were obtained for signaling in thin and thick areas of the same culture. Since a small percentage of neurons were GABAergic, we considered previously that a resultant small number of inhibitory synaptic connections formed within thinner cultures and that this reduced number was insufficient to regulate excitatory activity. These results are consistent with these prior considerations. The 3> fold increase in signaling following bicuculline addition of bicuculline to established cultures [32], coupled with only 25% of total neurons displaying GABA, suggests that inhibitory neurons control more > 1 excitatory neuron. These findings suggest that, on average, a single inhibitory neuron regulates the activity of 3 excitatory neurons. This is in line with prior demonstrations in a study that stated that inhibitory neurons control multiple excitatory neurons during development [34]. Multiple synaptic arrangements could mediate the ability of one inhibitory neuron to regulate the activity of multiple excitatory neurons (Fig. 6).


We routinely allowed astroglial proliferation in our ex vivo neuronal networks; we and others have demonstrated that factors released by glial cells increased complex signaling [28, 32, 35].
5. THE ROLE OF INHIBITORY NEURONS IN THE DEVELOPMENT, MATURATION, AND AGING OF THE NERVOUS SYSTEM IS RECAPITULATED IN EX VIVO NETWORKS
A developmental delay in cortical inhibitory activity is observed in situ. Whether or not this eventual appearance of inhibitory activity is derived from excitatory neurons or by the transient excitatory activity of GABAergic neurons during development is unclear. We utilized the reductionist approach afforded by ex vivo neuronal networks to examine this possibility.
Between 20–30 days in culture, individual signals (“spikes”) from 0.2–0.4 mV in amplitude appeared, which was followed by high amplitude epileptiform activity. Bursts (defined as clusters of ≥3 low-amplitude spikes within 0.7sec) remained following the decline of epileptiform activity (Fig. 7). Notably, the bicuculline had no effect on the initial signaling but subsequently fostered continued epileptiform activity (Fig. 7). This suggested that GABAergic neurons were functioning as inhibitory neurons at this point [36].
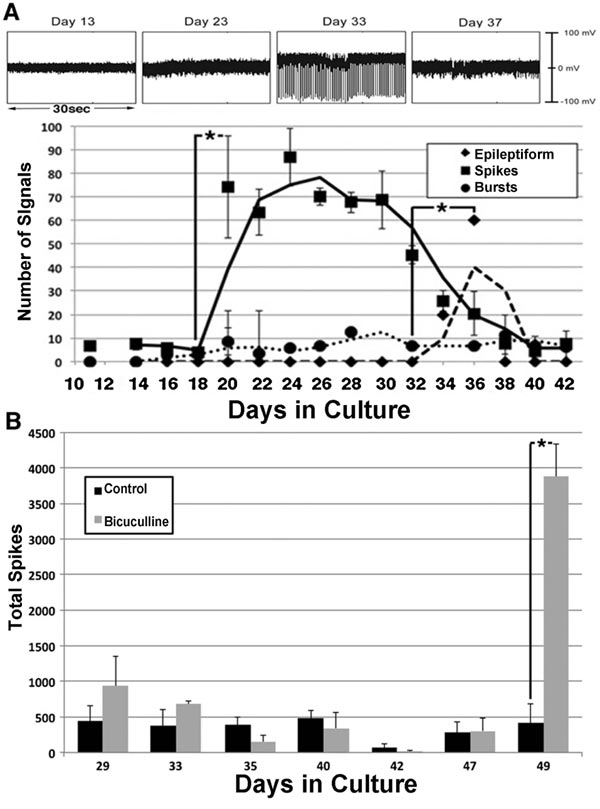
These results are consistent with the delayed formation of GABAergic synapses [37, 38], and with transient excitatory activity by newly-formed GABAergic synapses [39]. Additional studies have demonstrated an age-related decline in inhibitory neuronal activity [31, 40], which may contribute to the dysregulation of inhibitory activity and resultant aberrant excitatory activity observed in several age-related neurodegenerative conditions [40, 41].
This transient excitatory activity of GABAergic neurons also contributed to the level of total excitatory signaling required to trigger the developmental conversion of initially excitatory GABAergic neurons to inhibitory neurons [42]. Moreover, insufficient GABAergic excitatory activity actually inhibited this developmental transition. This leaves open the possibility that failure to sufficient GABAergic neurons to convert from excitatory to inhibitory could leave an individual at a threshold for seizures [42]. One interpretation of these latter findings is that epilepsy could arise not from excessive excitatory activity but rather from a lack of sufficient inhibitory activity. These latter findings underscore the ability of the reductionist approach of ex vivo neuronal networks to elucidate aspects of nervous system development and homeostasis that have remained difficult if not impossible to clarify in situ.
6. EX VIVO NEURONAL NETWORKS DISPLAY COMPLEX RESPONSES TO STIMULI
Rather than functioning in isolation, nervous systems from simple to complex benefit their host organism by receiving and processing information from the environment via sensory input. We and others took advantage of the ability to deliver electrical stimulation to networks via the MEA, which provides a model of sensory input.
A biphasic square wave signal utilizing the bath ground electrode as the distal grounding electrode was first applied Fig. (8A, left panel), which resulted in a 2.7-fold increase in signals from the network [43]. We wondered whether or not stimulation with more complex signals would foster the network to display a more complex response. We first applied the same biphasic square wave 3 times in rapid sequence (Fig. 8A), which induced a 1.5-fold increase in network signals versus those elicited by a single stimulation.
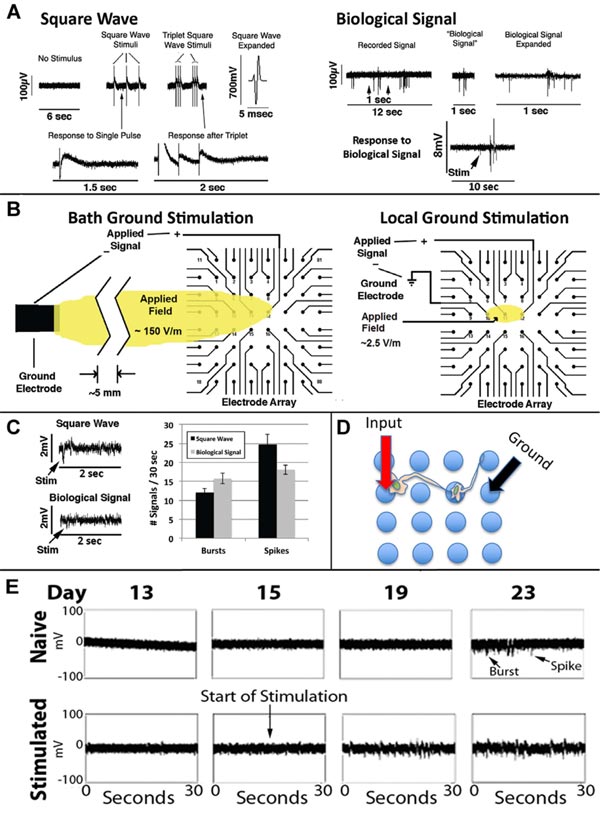
Since networks were not receiving any external stimulation spontaneously developed complex signaling patterns, including conversion of initially excitatory neurons to inhibitory neurons [27, 43], we considered that an actual neuronal signal might invoke even more complex responses than biphasic square waves. We, therefore, extracted a 1 sec portion from a recorded spontaneous signal from a mature network (defined as the “Biological Signal”; Fig. 8A, right panel). We amplified the Biological Signal to 1mV in amplitude, which invoked a response at least as robust as the above square wave signals that themselves were substantially larger in amplitude (700mV; Fig. 8A).
We then developed a method for localized stimulation of portions of the network, using 3 of the MEA electrodes (Fig. 8B).
We then compared localized stimulation of networks with the square wave and Biological Signal. The Biological Signal induced more bursts versus individual signals (p<0.03; Student’s t-test; Fig. 8C). We examined the consequences of early, localized stimulation with the Biological Signal on network development. Stimulation at day 15 hastened the appearance of both spikes and bursts (Fig. 8E) as well as the transient developmental epileptiform phase [36].
7. EX VIVO NEURONAL NETWORKS CAN UNDERGO LONG-TERM POTENTIATION
The ability of the nervous system to learn and establish memory evolves perhaps more than once because of survival pressures for increasingly complex organisms. Synaptic alteration, often referred to as long-term potentiation, is considered to be the basis for learning [44-50].
We previously demonstrated that network stimulation during development fostered an earlier appearance of mature signaling patterns (Fig. 8) [27, 36, 43]. We undertook to examine whether or not more extensive stimulation could invoke a long-term alteration in signals elaborated by ex vivo neuronal networks.
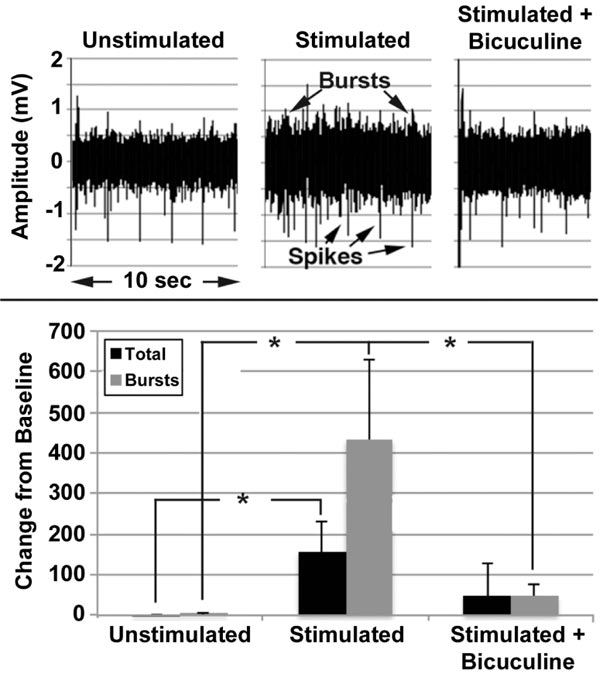
Mature networks (i.e., >30 days after plating) were repetitively stimulated for 5min, twice daily at 6hr intervals for 5 days in the presence and absence of bicuculline.
Unstimulated networks displayed a mixture of spikes and bursts (Fig. 9), with bursts accounting for approximately 35% of the total signals. No change was observed over 5 days in the absence of stimulation, but stimulation for 5 days increased total signals and bursts (Fig. 9). Bicuculline attenuated these increases. Increased signaling following stimulation indicates that mature networks can undergo synaptic plasticity, while prevention by bicuculline confirms a role for inhibitory neurons in this plasticity, consistent with findings in situ [52]. For reviews, see other research works as well [53-56].
8. MODELING OF MOTOR OUTPUT IN RESPONSE TO SENSORY INPUT IN EX VIVO NEURONAL NETWORKS
Since our networks responded to stimulation as a model of sensory input as described above, we designed a system that allowed this network to generate responsive output in terms of motor activity. The ex vivo network in this system functioned as interneurons – that is, essential as a central nervous system generating a motor response to sensory input.
The software interface can be obtained at https://github .com/ab3nd/NeuronRobotInterface.The ROS module (“img_ slicer”) (available at https://github.com/ ab3nd/NeuronRobot Interface/tree/master/catkin_ws/src/img_slicer/src) con-verted recorded video into HSV (Hue, Saturation, Value), and separated signals into hue, saturation, and value planes. The hue image was thresholded, and all pixels of the desired hue (red in this example) were converted to white, and all others converted to black (Fig. 10).
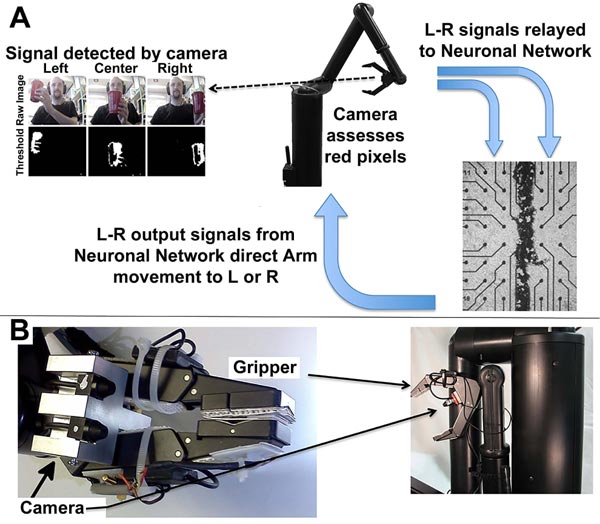
The field to be observed was separated into 5 segments while pixels summed and compared among these segments. If the majority of pixels were in the rightmost segments, the network was stimulated on its right side by the conversion of pixels into Biological Signals. This fostered increased synaptic signaling of the right portion of the network (Fig. 10). Resultant signals were converted to motion command for the Manus robotic arm by the ROS module, “act_vector” (https://github.com/ab3nd/NeuronRobotInterface/tree/master/catkin_ws/src/activation_vector/src) [57]. The requirement for software-mediated translation of pixels into digitized synaptic signals, followed by the translation of synaptic signals into motion commands to direct the Manus arm remain consistent with physiological conversion of photons into synaptic signals and subsequent conversion of synaptic signals to direct actin and myosin interactions that mediate muscle motility.
Network activity in response to camera input and target tracking is shown in Fig. (11).
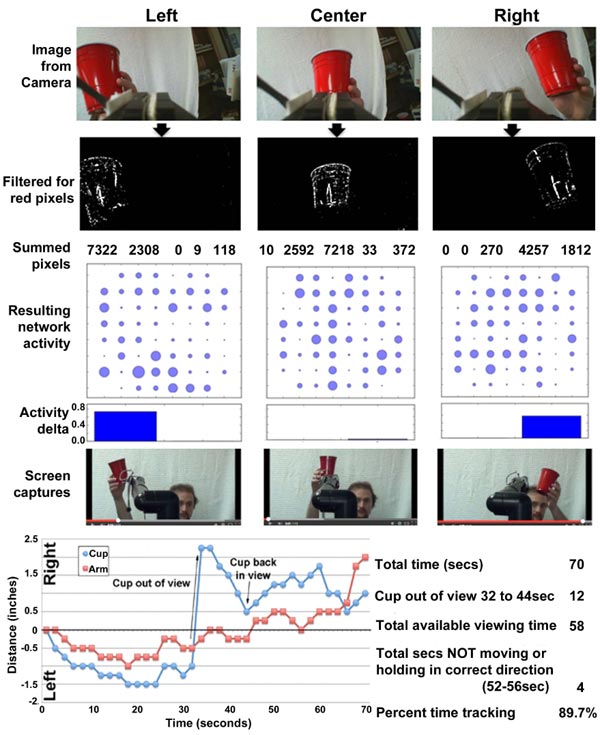
The robot arm accurately tracked the target (Fig. 11).
Previous studies have utilized similar ex vivo networks to operate a small model vehicle [58, 59].
9. RUDIMENTARY ASPECTS OF CONSCIOUSNESS ARE DISPLAYED IN EX VIVO NEURONAL NETWORKS
After decades of largely philosophical consideration, consciousness has more recently been studied as a physiological activity [1, 4, 60-65]. “Neural Correlates of Consciousness” is considered to be the physical cortical regions that generate aspects of consciousness [6, 10]. Notably, however, the development of the neocortex preceded humans and is present, albeit less well developed, in reptiles and mammals [5]. Aspects of consciousness are also observed in birds [6]. While more complex nervous systems are associated with more complex behaviors, it has been noted that behavioral complexity does not precisely correlate with nervous system complexity [60, 66]. If consciousness is an emergent property of cortical activity, it is reasonable to consider that animals display some aspects of consciousness. In this regard, it has been suggested that primitive emotions may underly the evolutionary development of consciousness and that aspects of consciousness are experienced by animals as well as humans [6-9].
Along with this, consciousness can be divided into primary consciousness (which encompasses relatively simple responses to the environment, adaptation, and long-term memory) and higher-order consciousness, which allows future planning, self-awareness, and complex language [11]. While higher-order consciousness may be restricted to humans and perhaps other primates [11], it is considered that the primary aspects of consciousness are widely present among animals [12, 13], which is consistent with the wide-spread distribution of general neuronal features among animals [14]. The neural activity underlying primary consciousness must still be present in animals with higher-order consciousness [11]. In this regard, ex vivo neuronal networks indicate multiple aspects of primary consciousness, including complex signaling regulated by the extent of synaptic connections, responsiveness to the environment including the appropriate direction of motor activity, and long-term potentiation (which is the cellular basis for learning and memory).
Loss of higher-order consciousness is directly linked to perturbation of neuronal interactions, supporting the notion that consciousness is an emergent property of neuronal interaction [6, 67]. Since the loss of organized synaptic activity is associated with disruption of consciousness, it is conceivable that the timing or synchronization of neural activity, rather than simply the overall level of spiking, might correlate with or mediate awareness [68]. Which region give rise to consciousness is not entirely clear and may involve the upper brain stem in addition to, and by interaction with, cerebral cortical regions, suggesting that relatively simple signaling contributes to consciousness [69, 70].In neurodegenerative conditions such as Alzheimer’s disease, following traumatic injury or administration of anesthesia, consciousness can be lost, yet portions of the cerebral cortex may still remain active with individuals persisting in a so-called “vegetative state” [71]. Notably, the same agents that perturb neuronal signaling leading to impaired consciousness in situ also perturbed neuronal signal in ex vivo neuronal networks, such as the beta-amyloid peptide that accumulates in Alzheimer’s disease [72-74], the neurotoxin tetrodotoxin, the GABAergic antagonist bicuculline, various excitatory antagonists [32, 33], and excess extracellular glutamate [75]. This is consistent with in situ studies suggesting the contribution of relatively simple neuronal interaction to consciousness [11, 76].
10. DISCUSSION
The nervous system allows an organism to receive and react to information from the environment. The nature and range of potential reactions increases in complexity with increasingly developed nervous systems. No matter how complex and diverse these reactions may be, they are ultimately derived from simple synaptic interaction of excitatory and inhibitory neurons as modulated by the architecture of the organism’s nervous system. This is underscored by studies with ex vivo neuronal networks. Our studies and those of other laboratories demonstrate that ex vivo neuronal networks can model multiple aspects of the nervous system, including development [42], maturation [36], long-term potentiation [43], environmental interaction [43, 57], aging [31] and ultimate neurodegeneration [72-75]. While the “default” interpretation of neuronal behavior is perhaps to consider stimulation as evoking response, our studies underscore the additional requirement of inhibitory neuronal activity for the achievement of both the development as well as maintenance of complex “responsive” actions of the nervous system. The reductionist approach of simple ex vivo neuronal networks may continue to provide insight into the complex functionality of nervous systems in situ.
Results obtained with ex vivo neuronal networks in several cases are paralleled with the results generated using computer-generated neural networks. This included our demonstration of the role of inhibitory neurons in complex signaling, development of the nervous system, and in an embodiment of networks with visual input and motor output [32, 33, 77]. This underscores the potential usefulness of ex vivo neuronal networks to probe the physiological basis of phenomena observed with computer-based neural networks.
11. GETTING STARTED WITH MEAS AND Ex Vivo NEURONAL NETWORKS
Multiple complete commercial MEA systems are available on a number of software and hardware platforms. Commercial systems, unfortunately, require a level of financial investment that can be prohibitive for laboratories that require extramural support since preliminary data justifying such support cannot be obtained in the absence of a computer-neuronal network interface. Our initial system used a custom 16-pin interface; details and images are presented in another study [28]. Despite the requirement of multiple relocations to access all channels, this simple system was capable of recording and stimulation [27]. We subsequently incorporated a 64-channel interface and developed a custom software program “Raptor” that provides a simplified user interface and can export to Excel (Fig. 12); this
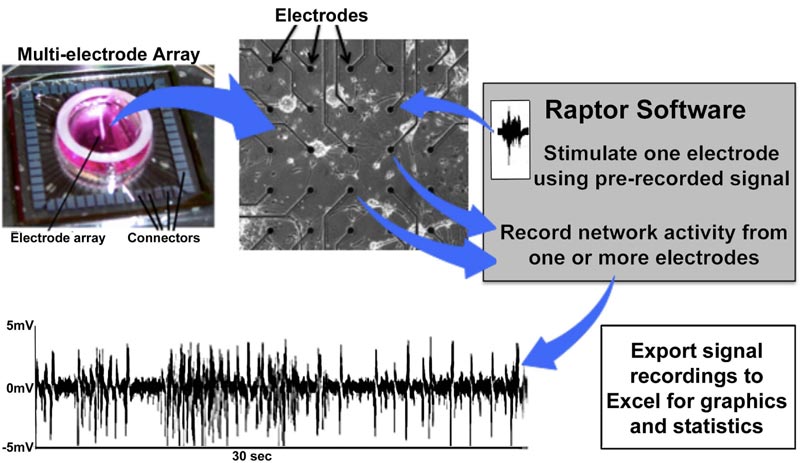
program, utilized for most of the studies presented herein, is freely available to all investigators (contact the author or visit https://github.com/mtgjbird/Raptor). Raptor requires LabView (National Instruments) run-time engine 2013 SP1 or later (http://www.ni.com/download/labview-run-time-engine-2013-sp1/4540/en/). Other appropriate software are available in different studies [78, 79]. Another low-cost MEA system that also utilizes LabView has been described previously [80].
CONCLUSION
Ex vivo neuronal networks established on MEAs provide reductionist models that allow analyses of normal development as well as for perturbations that accompany aging and other neuropathological conditions and for the screening of potentially therapeutic compounds [81]. Imbalance of excitatory and inhibitory neuronal activity, readily achievable in ex vivo networks, may be especially useful in studies of neurological disorders involving GABAergic dysregulation of, including epilepsy, schizophrenia, age-related neuropathic pain, Parkinson’s disease, and Alzheimer’s disease [40, 41]. Further study may elucidate other roles of inhibitory neuronal activity related to the normal and abnormal development and maintenance of the nervous system.
CONSENT FOR PUBLICATION
Not applicable.
FUNDING
This study has been sponsored by US Army Grants W911NF0810222, W911NF1010227, and W911NF1510206.
CONFLICT OF INTEREST
The authors declare no conflict of interest, financial or otherwise.
ACKNOWLEDGEMENTS
Figures and portions of the text presented in this review were taken from original publications sponsored by US Army Grants W911NF0810222, W911NF1010227, and W911N F1510206 to the author. The author is grateful for the continued advice of Dr. Elmar Schmeisser.
We thank the journals in which some of these figures appeared previously for granting permission to utilize figures, or portions of them, in this review. Each such figure has a reference in its legend to the appropriate source.


