RESEARCH ARTICLE
Geographic Distribution of the Prevalence of Neurocysticercosis in the Municipality of Piedade, São Paulo
José Augusto Camargo1, *, Reinaldo José Gianini1
Article Information
Identifiers and Pagination:
Year: 2021Volume: 15
First Page: 16
Last Page: 20
Publisher ID: TONEUJ-15-16
DOI: 10.2174/1874205X02115010016
Article History:
Received Date: 7/9/2020Revision Received Date: 8/2/2021
Acceptance Date: 16/2/2021
Electronic publication date: 16/04/2021
Collection year: 2021

open-access license: This is an open access article distributed under the terms of the Creative Commons Attribution 4.0 International Public License (CC-BY 4.0), a copy of which is available at: https://creativecommons.org/licenses/by/4.0/legalcode. This license permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Abstract
Background:
Neurocysticercosis is a globally occurring parasitosis that affects the central nervous system. The main clinical manifestation is seizures.The aim of this study was to analyze the geographic distribution of patients with seizures attending the Emergency Unit of the Piedade municipality, SP, with a diagnosis of neurocysticercosis, and to compare the association of its prevalence with age, basic sanitation and food hygiene habits between rural and urban areas.
Methods:
One hundred thirty-six patients with seizures were evaluated. The Epidemiological data of all subjects will be described.
Results:
The diagnosis of neurocysticercosis was confirmed in 24 patients, corresponding to a prevalence of 17.65%. The presence of an open sewer predominated in the rural area, while the consumption of unwashed fruits, presence of elderly people and geographic distribution were similar in the two areas.
Conclusions:
Urgent measures to raise awareness about the disease and preventive actions should be taken by world authorities.
1. INTRODUCTION
Neurocysticercosis (NCC) is a term used to define the involvement of the central nervous system (CNS) by the larval form of Taenia solium. This disease is the leading cause of epilepsy in adults worldwide and one of the main parasitosis associated with chronic morbidity on all continents. Other clinical manifestations observed are dementia, intracranial hypertension, headache and meningitis, among others. In its vesicular form, the cysticercus can persist for many years in the CNS without the patient developing symptoms [1-4].
Despite its importance and global morbidity, unfamiliarity of the population with NCC makes it an underestimated disease. The lack of investment in scientific research prevents the advances in the treatment and prevention of this neuroinfection that are necessary in order to help millions of patients with epilepsy. It is estimated that 50 million people in the world have NCC, with 50,000 annual deaths [3-8]. However, new research is necessary to update this number.
Brazil is a country that is greatly affected by the taeni- asis/cysticercosis complex. The lack of basic sanitation and precarious hygiene habits, including food hygiene, among other factors, contribute to the occurrence of this disease. Although one of the richest states in the country, São Paulo has the same problem in all regions, regardless of the socioeco- nomic situation. One of the few studies on the topic available in Brazil reports an incidence of 54 NCC cases per 100,000 inhabitants in Ribeirão Preto, located in the northeastern region of the state [1, 3, 5]. Within this context, the aim of this study was to analyze the geographic distribution of the prevalence of NCC in the municipality of Piedade, São Paulo.
2. MATERIALS AND METHODS
2.1. Patients
A cross-sectional study was conducted on 136 patients of both genders with a history of seizures. The subjects were referred to the responsible researcher by physicians of the emergency department for neurological evaluation. Neurocys- ticercosis was diagnosed in 24 patients according to the criteria of Del Bruto et al. [6]
2.2. Study Area and Period
Piedade (São Paulo) is known as the onion capital because it is a large producer in Brazil. Today, the municipality’s agriculture is diverse, producing fruits, vegetables, artichokes, strawberries, and onions. The municipality is located between mountains, within the Serra do Mar, in an ecological preservation area, and comprises an area of 745.52 km2. The population mainly consists of native peoples and the immigration of Italians and Japanese, among other nationa- lities, only occurred in the middle of the 20th century. The total population is 52,214 inhabitants; of these, 23,782 live in the urban area and 28,432 in the rural area, including 26,602 men and 25,612 women (Source: 2010 Census/IBGE).
The study was conducted from May 2014 to August 2015 and included all residents in the urban and rural areas of the municipality.
2.3. Data Collection
A questionnaire was developed and pre-tested according to the objectives of the study. The data were collected by the responsible researchers through personal interview. The participants received information about the study and its objectives and that their names would be identified by initials or surnames to maintain data confidentiality. Children younger than 12 years and pregnant women were excluded from the study. For participants younger than 18 years, the questionnaire was applied in the presence of the parents or legal representative. All participants were submitted to cranial tomography and/or magnetic resonance imaging.
2.4. Ethical Approval
The study was approved by the Ethics Committee of the University of São Paulo (2013/1068-FMUSP).
2.5. Statistical Analysis
All data of the variables studied were entered into the Excel program and subsequently transferred to the STATA 16.1 program. The chi-squared test or Fisher’s exact test was applied to analyze the association between the variables studied and the presence of NCC [7]. Prevalence ratios and the respective 95% confidence intervals were calculated using Poisson regression in both univariate and multivariate analysis. A p value <0.05 was considered significant.
For the analysis of spatial distribution, geocoding of the addresses was performed as follows: 1. The Google app My Maps was used for georeferencing. The addresses listed in an Excel file were geocoded following an accuracy rating. 2. The necessary corrections were made to locate the non-geocoded cases. 3. Based on the list with the coordinates of the points, the file was transferred to MapInfo (version 10) for spatial analysis. 4. We used the census sector mesh for the city of Piedade (IBGE, 2010). The layers and the spreadsheet containing the cases then permitted to identify the number of cases residing in the urban and rural area of the municipality. In addition, we classified the NCC-positive and -negative cases according to the area of residence.
3. RESULTS
The questionnaire was applied to 136 patients, including 54 (39.71%) men and 82 (60.29%) women ranging from 13 to 86 years. All patients with NCC, 24 (17.65%), had the calcified cerebral form of the disease. In this group in which the parasite was detected by imaging examinations, 29.2% lived in the urban area and 70.8% in the rural area. There were 29.2% men and 70.8% women.
All respondents, 136 (100%), reported to have heard, at some point, comments about “pork disease”. The participants knew that, in this disease, the parasite can lodge in the human brain and that after the death of the parasite, also based on the information of medical professionals, the disease will cease without causing future problems for the organism.
As can be seen in Table 1, there was a significant difference in age and NCC+ in the urban area due to the higher prevalence among older adults. A higher prevalence of the open sewer category was observed in rural area. Comparison of the urban and rural areas showed a lower prevalence of NCC+ in the rural area for public sewer/septic tank, for water-/urban untreated, urban treated but not filtered, and for fruits-/washed (filter water or boiled or chlorinated or vinegar). In addition, analysis of the total sample in the rural area revealed a lower prevalence of NCC+ when compared to the urban area (p<0.01).
|
Variable/ category |
Urban | - | - | - | Rural | - | - | - | Rural/urban |
|---|---|---|---|---|---|---|---|---|---|
| - | NCC- | NCC+ | p | 95%CI | NCC- | NCC+ | p | 95%CI | 95%CI |
| Age (years) | - | - | 0.02 | - | - | - | 0.82 | - | - |
| 13-30 | 16 | 3 | - | 1 | 17 | 2 | - | - | 0.67(0.11-4.00) |
| 31-45 | 20 | 3 | - | 0.83(0.17-4.1) | 18 | 1 | - | 0.50(0.05-5.51) | 0.40(0.04-3.85) |
| 46-60 | 15 | 4 | - | 1.33(0.30-5.96) | 11 | 1 | - | 0.79(0.07-8.73) | 0.40(0.04-3.57) |
| 61 or more | 9 | 10 | - | 3.33(0.92-12.1) | 6 | 0 | - | 0 | 0 |
| - | - | - | - | - | - | - | - | - | - |
| Sewage disposal | - | - | 0.41 | - | - | - | <0.01 | - | - |
| Public sewer or septic tank | 59 | 19 | - | 1 | 52 | 2 | - | 1 | 0.15(0.04-0.63) |
| Open sewer | 1 | 1 | - | 2.05(0.27-15.3) | 0 | 2 | - | 27(3.8-191.7) | 2(0.5-8) |
| - | - | - | - | - | - | - | - | - | - |
| Drinking water | - | - | 0.12 | - | - | - | 0.69 | - | - |
| Urban treated. filtered | 12 | 1 | - | 0.27(0.04-1.85) | 2** | 0 | - | 0 | 0 |
| Urban untreated. Urban treated but unfiltered. and rural | 48 | 19 | - | 1 | 50 | 4 | - | 1 | 0.26(0.09-0.72) |
| - | - | - | - | - | - | - | - | - | - |
| Fruit and vegetable consumption | - | - | 0.25 | - | - | - | 0.78 | - | - |
| Washed (filtered or boiled or chlorinated water or vinegar) | 56 | 17 | - | 1 | 51 | 4 | - | 1 | 0.31(0.11-0.88) |
| Unwashed | 4 | 3 | - | 1.84(0.71-4.8) | 1 | 0 | - | 0 | 0 |
| - | - | - | - | - | - | - | - | - | - |
| Total sample | 60 | 20 | - | - | 52 | 4 | - | - | 0.29(0.10-0.90) |
Table 2 shows a significantly higher prevalence of NCC+, regardless of the other factors of the table, for older adults in the urban area and total sample; for the open sewer category in the total sample, and for unwashed fruits in the urban area and total sample. When adjusted for the other variables, the prevalence of NCC did not differ significantly between the rural and urban areas (p=0.06).
Fig. (1) illustrates the concentration of NCC+ cases in the urban area.
4. DISCUSSION
The prevalence of NCC in the sample was 17.65%, which was strongly associated with age above 60 years in both areas, the presence of open sewers, especially in the rural area, the lack of drinking water treatment, particularly in the urban area, treated water but without adequate filtration in the urban area, and inappropriate washing of fruits and vegetables before consumption in both areas.
Analysis of the geospatial distribution of NCC showed a larger proportion of cases in the urban area; however, there was no significant difference between the rural and urban areas. The hypotheses to explain this result include the natural history of the disease, rural-urban immigration, and the site of exposure differing from the place of residence, among others. Within this context, the following factors are very important for the eradication of the taeniasis/cysticercosis complex: personal hygiene habits, care with the preparation and washing of foods before consumption, appropriate drinking water treatment including filtration, investment in basic sanitation, adequate preparation of pork products, and rigorous monitoring of pig farm facilities and commercialization by health surveillance authorities.
| Variable/category | Urban | - | - | Total* | - | - |
|---|---|---|---|---|---|---|
| - | PR | 95%CI | p | PR | 95%CI | p |
| Age (years) | - | - | - | - | - | - |
| 13-30 | 1 | - | - | 1 | - | - |
| 31-45 | 0.96 | 0.16-5.79 | 0.96 | 0.90 | 0.22-3.72 | 0.06 |
| 46-60 | 2.36 | 0.38-14.5 | 0.36 | 1.80 | 0.47-7.00 | 0.89 |
| 61 or more | 5.89 | 1.14-30.3 | 0.03 | 4.54 | 1.29-16.03 | 0.02 |
| - | - | - | - | - | - | - |
| Sewage disposal | - | - | - | - | - | - |
| Public sewer or septic tank | 1 | - | - | 1 | - | - |
| Open sewer | 5.34 | 0.44-64.2 | 0.19 | 9.29 | 2.25-38.4 | 0.002 |
| - | - | - | - | - | - | - |
| Drinking water | - | - | - | - | - | - |
| Urban treated. filtered | 0.13 | 0.01-1.20 | 0.08 | 0.12 | 0.01-1.20 | 0.07 |
| Urban untreated. Urban treated but unfiltered. and rural | 1 | - | - | 1 | - | - |
| - | - | - | - | - | - | - |
| Fruit and vegetable consumption | - | - | - | - | - | - |
| Washed (filtered or boiled or chlorinated water or vinegar) | 1 | - | - | 1 | - | - |
| Unwashed | 7.97 | 1.47-43.1 | 0.02 | 7.03 | 1.49-33.2 | 0.01 |
| - | - | - | - | - | - | - |
| Rural/urban area | - | - | - | 0.34 | 0.11-1.04 | 0.06 |
| - | - | - | - | - | - | - |
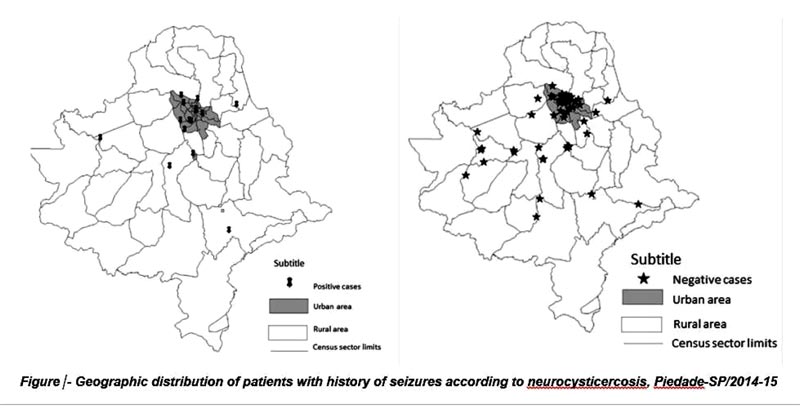 |
Fig. (1). Spatial distribution of seizure cases positive and negative for neurocysticercosis in the municipality of Piedade, São Paulo. |
The results found in this study make a valuable contribution since the prevalence of NCC in Brazil is unknown because of the absence of compulsory notification in many states and the difficulty of access of the majority of the population to diagnostic methods. The calcified forms of the parasite found in the patients studied may pose a serious health threat because they can cause irreversible changes in the individual and require lifelong treatment and medications. The diagnosis of NCC is made by a combination of immunological methods and neuroimaging [6, 8-13]. The false notion evidenced in the population studied, similar to that observed in the world population, that the calcified form of the parasite does not cause health damage in affected individuals must be urgently revised and corrected [3, 4, 8, 9, 11-14]. According to Camargo et al [3, 4, 15, 18-20], the expressions cerebral calcification, inactive cyst, dead parasite, cysticercotic calcification and dead cysticercus, which are all very common in popular language, must be revised and adequately explained to the population and health professionals who are unaware of the disease. In the area studied, other asymptomatic individuals may be contaminated with different forms of the parasite, which can lodge in the CNS and persist for many years without causing symptoms [1, 2, 8, 9, 16, 17].
As mentioned earlier, the factors that contribute to the endemic appearance of the taeniasis/cysticercosis complex are numerous and closely related to personal and family hygiene habits and environmental factors. Within this context, government investment in health and educational programs is needed, advising the population on the importance of controlling and preventing this disease in view of its severe consequences for public health and the lack of access of the majority of the population to diagnostic methods [21-25].
CONCLUSION
Considering the parasite’s biological cycle, avoiding the deposition of human feces in septic tanks may be an important start for disease control. Raising the awareness of the population and of the competent authorities about the disease and methods for its prevention is an urgent measure, not only for the area studied but for the world population living in urban and rural regions.
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
The study was approved by the Ethics Committee of the University of São Paulo, Brazil (2013/1068-FMUSP).
HUMAN AND ANIMAL RIGHTS
No animals were used in this research. All human research procedures were followed in accordance with the ethical standards of the committee responsible for human experimentation (institutional and national), and with the Helsinki Declaration of 1975, as revised in 2013.
CONSENT FOR PUBLICATION
Informed consent was obtained from all participants.
AVAILABILITY OF DATA AND MATERIALS
Not applicable.
FUNDING
None.
CONFLICT OF INTEREST
Dr. José Augusto Camargo is the Editorial Board Member of The Open Neurology Journal.
ACKNOWLEDGEMENTS
Declared none.








