All published articles of this journal are available on ScienceDirect.
Chronic Lyme Disease and Co-infections: Differential Diagnosis
Abstract
In Lyme disease concurrent infections frequently occur. The clinical and pathological impact of co-infections was first recognized in the 1990th, i.e. approximately ten years after the discovery of Lyme disease. Their pathological synergism can exacerbate Lyme disease or induce similar disease manifestations. Co-infecting agents can be transmitted together with Borrelia burgdorferi by tick bite resulting in multiple infections but a fraction of co-infections occur independently of tick bite. Clinically relevant co-infections are caused by Bartonella species, Yersinia enterocolitica, Chlamydophila pneumoniae, Chlamydia trachomatis, and Mycoplasma pneumoniae. In contrast to the USA, human granulocytic anaplasmosis (HGA) and babesiosis are not of major importance in Europe. Infections caused by these pathogens in patients not infected by Borrelia burgdorferi can result in clinical symptoms similar to those occurring in Lyme disease. This applies particularly to infections caused by Bartonella henselae, Yersinia enterocolitica, and Mycoplasma pneumoniae. Chlamydia trachomatis primarily causes polyarthritis. Chlamydophila pneumoniae not only causes arthritis but also affects the nervous system and the heart, which renders the differential diagnosis difficult. The diagnosis is even more complex when co-infections occur in association with Lyme disease. Treatment recommendations are based on individual expert opinions. In antibiotic therapy, the use of third generation cephalosporins should only be considered in cases of Lyme disease. The same applies to carbapenems, which however are used occasionally in infections caused by Yersinia enterocolitica. For the remaining infections predominantly tetracyclines and macrolides are used. Quinolones are for alternative treatment, particularly gemifloxacin. For Bartonella henselae, Chlamydia trachomatis, and Chlamydophila pneumoniae the combination with rifampicin is recommended. Erythromycin is the drug of choice for Campylobacter jejuni.
INTRODUCTION
In Lyme disease, other infections, whose pathological synergism exacerbate the disease or induce similar clinical manifestations, can exist concurrently. Such concomitant infections are termed co-infections. Co-infections can be transmitted together with Borrelia burgdorferi by tick-bite, and result in multiple infection. Part of co-infections is independent of tick-bite.
The goal of this review was to summarize the more important co-infections completed with some personal experiences and with a short summary on reactive arthritis. Because of the similarity of the clinical symptoms of tularemia, Q fever, parvovirus B19 and Campylobacter jejuni infections to those of Lyme disease a short summary of these infections are also included.
RELEVANT CO-INFECTIONS IN LYME DISEASE
Co-infections can exacerbate Lyme disease through immune system modulation and are considered to be the major cause for resistance to therapy [1-17]. The importance of co-infections in the disease process, i.e. their pathogenicity compared to Lyme disease, has not been clarified. In cases with double or multiple infections, to determine which infection predominates in the pathological process is difficult. There are substantial overlaps between the clinical symptoms caused by co-infections and Lyme disease. Consequently, an unequivocal assignment of the manifestations of the disease to existing infections might be difficult. The diagnostic difficulties of Lyme disease and co-infections always concern chronic Lyme disease (late Lyme disease, stage III). The synergic-pathological mechanism requires that co-infections are also present in chronic persistent form. Anamnestic consideration of the acute form of co-infections may be helpful to recognize their persistence in the chronic stage.
For the majority of co-infections, as for Lyme disease, laboratory diagnostic tests for indirect pathogen detection, including serological tests and lymphocyte transformation test (LTT, syn. LPT (lymphocyte proliferation test)) are available. Previous infection can be confirmed with serological tests, but a positive serological finding is not proof that the infection causes the current illness. It cannot prove the presence of active infection, and in case of seronegativity it cannot exclude it. Only if the occurrence of positive laboratory findings or their deterioration occur in temporal relationship with the disease state and development assumption of chronic disease may be justified e.g. espacialy in cases with previous sero-negativity or negative LTT or significancy lower initial values.
The significant co-infections in Lyme disease are caused by various Bartonella species, primarily Bartonella henselae, by Chlamydia trachomatis, Chlamydophila pneumoniae, Yersinia enterocolitica, and Mycoplasma pneumoniae (Table 1)
Co-infections in Lyme Disease
| Disease* | Pathogen | Mode of transmission | Reservoir | IC, EC |
|---|---|---|---|---|
| Tick-borne co-infections | ||||
| HGA (HGE) HME |
Anaplasma
phagocytophila E. chaffeensis |
Ticks Ixodes ricinus (Europe) Ixodes scapularis (USA) | White-
footed mouse (HGA) Red deer, human beings (HME) |
IC |
| Bartonellosis |
Bartonella henselae (Bartonella quintana, Bartonella bacilliformis) [18-20] |
Bite or
scratch wounds of dog or cat (saliva, claws); cat fleas; lice (B.
quintana) [21, 22], tick bite Others: dust mites, flea bites, flea feces (oral infection), contact with cats, contact with dogs (paws, saliva, lice [21], flies, gadflies, blood transmission, mother-child transmission [23] |
Cats [24-27], domestic and wild animals [28] | IC |
| Rickettsiosis Mediterranian spotted fever | Rickettsia helvetica Rickettsia conorii | Ticks, mites, fleas, lice | Ticks | IC |
| Tularemia | Francisella tularensis | Mosquitos, gadflies, fleas, lice, mites, oral, inhalation | Various vertebrates | IC |
| Q fever | Coxiella burnetii | Oral or inhalation | Cattle, milk, human beings | IC |
| Babesiosis | Babesia microti, Babesia divergens [29,30] | Ixodes ricinus (Europe), Ixodes scapularis (USA) [29, 30], blood transfusions [31], perinatal [32, 33] | Cattle (other vertebrates) | IC |
| Other co-infections (not tick-borne) | ||||
| Mycoplasma infections | Mycoplasma pneumoniae | Droplet infection, humans | Human beings | EC |
| Chlamydophila pneumoniae infection | Chlamydophila pneumoniae | Droplet infection, person to person | Human beings | IC&EC |
| Chlamydia trachomatis infection | Chlamydia trachomatis | Sexual contact | Human beings | IC&EC |
| Yersiniosis |
Yersinia
enterocolitica Yersinia pseudotuberculosis (USA) |
Fecal-oral | Various vertebrates | IC&EC |
| Parvovirus B19 infection | Human parvovirus B19 | Respiratory tract: droplet infection, person to person, during pregnancy, blood transfusion | Human beings | IC |
| Campylobacter jejuni infection | Campylobacter jejuni | Fecal-oral Game and domestic animals, particularly poultry, animal products, contaminated water |
Vertebrates | IC&EC |
IC = Intracellular; HGA = : human granulocytic anaplasmosis ; HGE = human granulocytic erlichiosis;
* = Relevant co-infections are highlighted in bold.
CO-INFECTIONS IN LYME DISEASE: OWN EXPERIENCE
The frequency of seropositivity and positive LTT of coinfections was evaluated. The laboratory examinations were done in the Institut für Medizinische Diagnostik (IMD), Berlin, Germany. For details of the methods used see reference [34]. The results are illustrated in Table 2. CD57 NK cells are frequently diminished in chronic Lyme disease, but seldom in cases involving only the so called HGE co-infections. The basic principle is that CD57 NK cells can be diminished in all chronic infectious diseases, but the phenomenon is observed relatively frequently in chronic LD.
Positive Serology and Positive LTT for Co-infections (%) in Patients with Chronic Lyme Disease, N = 108
| Pathogen | N | Positive Serology (%) | Positive LTT (%) |
|---|---|---|---|
| Mycoplasma pneumoniae | 36 | 36 | Nd |
| Chlamydophila pneumoniae | 66 | 62 | 66 |
| Chlamydia trachomatis | 100 | 5 | 100 |
| Yersinia enterocolitica | 58 | 58 | 50 |
| Bartonella henselae | 78 | 78 | Nd |
LTT = lymphocytic transformation test; Nd = not done, LTT=LPT (lymphocyte proliferation test).
In contrast to the USA, human granulocytic anaplasmosis (HGA) or human granulocytic ehrlichiosis (HGE) and babesiosis are of little importance as co-infections in Europe.
BARTONELLOSIS
Bartonellosis can be expected to have substantial significance as a Lyme disease co-infection. With regard to the health policy aspect, Lyme disease is more important because of its frequency. However, in this context it should be noted that bartonellosis has not been nearly as intensively investigated as Lyme disease. With the increasing development of laboratory tests, one may expect that the currently underestimated prevalence of bartonellosis will be more correctly registered in the future. The importance of this disease will also be determined on the basis of its frequency. My own observations also show that the serology for Bartonella is frequently positive in patients with chronic Lyme disease.
All facets of the transmission mode of bartonellosis have not yet been clarified. The most important data reported in the scientific literature are summarized in Table 1.
Bartonellosis (caused by Bartonella henselae and Bartonella bacilliformis) can be associated with a high variety of symptoms (Table 3). The bacterial inflammatory skin infection (scratch or bite location) is in no way obligatory, i.e. bartonellosis can also occur without the typical cat scratch disease, which is characterized by infected skin lesion and lymph node swelling. The main manifestations of the disease comprise in addition to infected skin lesion and swollen lymph nodes various multi-organ disorders (e.g. liver, spleen, nervous system, eye) [18-19], cf. Table 3.
Main Disease Manifestations of Bartonellosis
| Infected scratch or bite wound (cat, dog) tick bite, loose infestation and other infection (Table 1) |
| Lymph node swelling (regional or generalized [35] |
| Persistent fever of unknown origin |
| Abdominal pains, loss of weight [22] |
| Various eye disorders [36] |
| Neuroretinitis [37-39] |
| Neurological manifestations [40-42] Encephalopathy (very frequent) Transverse myelitis Neuroradiculitis Cerebellar ataxia Cerebral seizures [41] Cerebral infarcts due to vasculitis [41] CSF: mild mononuclear pleocytosis [40] EEG pathological |
| Musculoskeletal complaints [43] |
| Arthritis |
| Arthralgias |
| Myalgias |
| Tendinitis, chronic course of arthropathies [43,44] |
| Fatigue [45] |
Until 1993 only B. bacilliformis was known. The different Bartonella subspecies were first described and their pathological significance recognized in 1993 [22].
There are numerous overlaps with Lyme disease in the clinical manifestations of bartonellosis [46]. The laboratory diagnosis for bartonellosis is based on the analysis of blood smear, on the serology, on pathogen detection using culture methods and PCR and on the histopathological investigations. In early stage the laboratory findings show elevated erythrocyte sedimentation rate (ESR) and C-reactive protein (CRP) level as well as hypercalcemia.
In cases of infection with Bartonella, the blood smear initially shows pathogens on the outer membranes of erythrocytes, and in the further course of the disease the pathogens are increasingly localized intracellularly. In the process, the light colored center of erythrocytes is lost (Fig. 1).
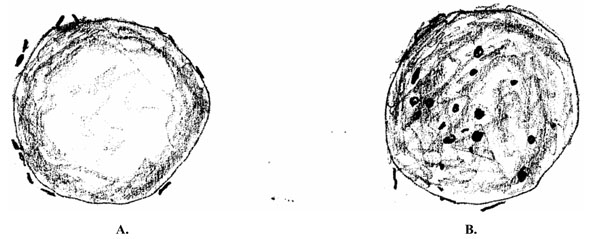
Erythrocytes infected with Bartonella henselae. A. At an early stage, the microorganism lies outside of the erythrocytes; B. With increasing duration of infection, the bacterium is primarily intracellular and erythrocytes loose their light-colored center.
There is no information on the value of serology in the literature. In particular, the question as to whether seronegativity rules out the disease has not been clarified. On the other hand, as it is the case for many other infectious diseases, a positive serological finding merely proves that an infection took place, but does not indicate active disease.
Detecting Bartonella in culture is difficult, and the sensitivity is very low, therefore this method of investigation is not part of the routine diagnostics. Detection of pathogens using PCR in biopsies appears to be promising [47, 48], but PCR analysis must follow the biopsy nearly immediately [49].
Chronic course of bartonellosis has been described in numerous studies, including in larger population studies [50-54]. The long duration of the disease frequently for several years, and the similar disease manifestations, makes it difficult to distinguish bartonellosis from chronic Lyme disease. Thus, bartonellosis is of great importance in the differential diagnosis of Lyme disease. In this context, attention should be paid to the fact that Bartonella henselae has been found in ticks and that transmission of Bartonella henselae by ticks to human has been documented by detecting the pathogen in the CSF [55]. Additionally, the prevalence of Bartonella henselae in ticks is apparently high; scientific studies determined a prevalence of 40% [56]. According to my own surveys, 78% of the patients with chronic Lyme disease proved to be seropositive for Bartonella henselae (Table 2).
Bartonellosis is accompanied by a stimulation of blood vessel formation, inducing vascularized tumors or granu-lomas in various regions of the skin (bacillary angiomatosis), in the liver (peliosis hepatis) or in the spleen (peliosis splenitis) [57-59]. These vascular tumors or granulomas exhibit a pathological sprouting of capillaries with hyperplasic and hyperproliferative endothelial cells [60]. In addition to angiomatosis, Bartonellosis also results in various other skin manifestations associated with increased vessel formation [23], therefore the determination of vascular endothelial growth factor (VEGF) in blood could be of diagnostic importance [23].
In all pathogenic Bartonellae (Bartonella quintana, Bartonella henselae, Bartonella bacilliformis) this effect on endothelial cells and on the induction of angiogenesis has been demonstrated. The vascular proliferation was primarily traced back to three factors [61-67], namely to elevated endothelial cell proliferation, to inhibition of endothelial cell apoptosis and to increased secretion of vasculo-proliferative cytokines. All these studies support the significant role of VEGF in Bartonella-induced endothelial cell proliferation [67].
Bartonellae are localized in erythrocytes and cause the deformation of erythrocyte membrane [68, 69]. The visualization of bartonellae in erythrocytes is used as a diagnostic tool, particularly with regard to the extent of infection [69]. However, irrefutable literature on erythrocyte infestation in chronic bartonelloses does not exist. The diagnostic value of a new method detecting the pathogen by means of cilia in blood smears cannot be assessed yet. The formation of intracellular blebs subsequent to the penetration of the organism into endothelial cells has also been showed for Bartonella quintana, i.e. a similar process to that observed on Borrelia burgdorferi in Lyme disease. Borrelia burgdorferi also has a high affinity for endothelial cells, and the development of blebs, particularly in chronic disorders, has been described. In connection with Lyme disease, the intracellular presence of the pathogen and the formation of biologically less active and more resistant forms (cysts, blebs) have been discussed as the cause of the failure of antibiotic treatment. Corresponding Additional parallels have been also found between Lyme disease and bartonellosis [cf.70-75]..
Two additional forms of bartonellosis merit to be noticed namely, the Oroya fever or Verruga peruana and the trench fever. Oroya fever and its recognition as an infectious disease date back to Carrion, who verified that the disease is infectious in a fatal self-test at the end of the 19th century. Trench fever was discovered at the beginning of the 20th century. Its transmission occurs via lice [77-79]. In 2002 the pathogen was detected for the first time in erythrocytes; as a result the transmission by lice became plausible [21]. Oroya fever and Verruga peruana are both Bartonella bacilliformis infections transmitted by sand flies. The disease occurs in the Andes. The acute form affects tourists who are immunologically naive with respect to Bartonella bacilliformis. Without treatment the mortality rate is 40%. To date, the factor, which influences the severe disease course is unknown.
Adequate studies are missing for the treatment of bartonellosis. There is no single treatment approved by the US Food and Drug Administration (FDA), the Center for Disease Control (CDC) or the Infectious Diseases Society of America (IDSA) [23]. This applies particularly for the chronic courses of the disease [80]. The use of the following antibiotics are recommended (Table 5): azithormycin [81,82], rifampicin, ciprofloxacin, trimethoprim combined with sulfamethoxazole, gentamycin [83,84], gentamycin i.v. [85], and doxycycline combined with gentamycin [86, 87]. This treatment is based, in part, on expert recommendations [88]. Further Other references are listed in Table 5.
Symptomatology of Bartonellosis
| Infected Scratch or Bite Wounds | |
|---|---|
| Lymphadenopathy | Frequently cardinal symptom |
| General symptoms | Fever, fatigue, drowsiness, sleep disorders, obesity, swelling in different parts of the body, weariness, headaches, air hunger, fainting fits |
| Encephalopathy | Cognitive, disorders, concentration and memory difficulties, blockage of thought processes, dyslexia and dysgraphia |
| Mental disorder | Depression, irritability, disturbed impulse control, agitation / aggression, panic attacks |
| Nervous system | Encephalitis, myelitis, neuralgias, muscular asthenia, paresthesias, neuroradiculitis, seizures, cerebral infarction, Guillain-Barré syndrome |
| Musculoskeletal system | Arthritis, arthralgias, myalgias, tendinitis, osteomyelitis, myospasm |
| Abdomen | Abdominal pain, hepatopathy (peliosis hepatis, hepatomegaly), splenopathy (peliosis splenitis), hepatic and splenic abscesses |
| Heart / Thorax | Endocarditis, pneumonia, pleural effusion, myocarditis |
| Eye | Oculoglandular disorder, conjunctivitis, neuroretinitis, papillitis, optic neuritis, retinochoroiditis, uveitis anterior, intermedia and posterior [76], acute maculopathy, choroiditis |
| Urogenital system | Bladder disorder, renopathy, genital disorders |
| Skin | Bacillary angiomatosis, striae, papulre edema (particularly of the feet), acne, occurrence of venous vessels at an unusual location, hyper- or hypopigmented skin, pea-sized pigment spots, Burgundy-colored, thin skin, Lesions of oral mucosa, Morphea, patchy hair loss, loss of eyelashes, change in hair color in hypopigmented areas, diffuse patchy exanthema, signs of hypervascularity, hematoma-like changes, skin lesions with indentation, erythema nodosum |
| Other | Parotid swelling, phlegmonous abscess in the neck region, septic shock, thrombocytopenic purpura, overproduction of calcitriol |
| Laboratory findings | Elevated ESR and CRP, hypercalcemia |
Antibiotic Treatment of Bartonellosis
| Antibiotic | Dose / Day | References |
|---|---|---|
| Azithormycin | 500 mg | [81, 82, 90-96] |
| Clarithromycin | 1000 mg | [89, 91-97] |
| Telithromycin | 800 mg | [89] |
| Rifampicin | 600 mg | [84, 89, 90, 91, 93, 95, 96, 98] |
| Trimethoprim + sulfamethoxazole | 875 / 125 mg 2x daily | [84, 92, 94, 98] |
| Ciprofloxacin | 1000 mg | [84, 89, 92-94, 98] |
| Doxycycline | 400 mg | [86, 89, 90, 91, 94-96] |
| Minocycline | 200-300 mg | [93] |
| (Other recommendations: erythromycin, roxithromycin, penicillin G, sparfloxacin, chloramphenicol, streptomycin, gentamycin, Augmentin, ticarcillin, cefotaxime, ceftriaxone, meropenem, trimethoprim and sulfamethoxazole. The information in the various publications is very contradictory. This is particularly true for gentamycin. Beta-lactam antibiotics do not act intracellularly and are therefore not suitable for the treatment of bartonellosis (author’s comment) [84,85,87,88,90,91,93,94,97,98-102]. There are substantial discrepancies between in vitro findings and in vivo efficacy. | ||
| Duration of
treatment (no reliable data basis) Acute early phase |
2 weeks | |
| Chronic course | 2 - 3 months |
The discrepancy between the in vitro findings and the in vivo results is set out in various publications [93,97,99, 102,103].
Since Bartonella henselae is primarily localized intracellularly in vivo, only antibiotics that act intracellularly are used; they are listed in Table 5.
Because instances of non-response and recidivism are not seldom in cases of chronic bartonellosis, long-term antibiotic treatment is recommended [89,99,102-104].
The efficacy of aminoglycosides (particularly gentamycin) is extremely controversially assessed. Publications with a positive assessment [92,94,95,99,102] are in opposition to other contributions, which assess aminoglycosides as ineffective or insufficiently effective [90,91,93].
Chlamydophila pneumoniae
Chlamydophila pneumoniae is important in the differential diagnosis of Lyme disease because it can cause nervous system disorders, myocarditis and reactive arthritis.
Chlamydias have special microbiological characteristics. The size of the pathogen is very small compared to other bacteria. Reproduction occurs within host cells and the pathogen is dependent on host cell ATP as it is not capable to produce its own. The pathogen exhibits two phenotypes, the elementary bodies and the reticulate bodies. The elementary bodies can exist extracellularly and represent the infectious form. Reproduction of the elementary bodies is only possible inside of host cells. Host cells phagocytize the elementary bodies, which intracellularly transform into reticulate bodies and undergo division. The elementary bodies are thus infectious, and the reticulate bodies reproductive. Some reticulate bodies can change back into elementary bodies, which are released subsequently to host cell lysis. The released elementary bodies infest then further host cells. Consequently, precondition for an adequate treatment is both the intra- and extracellular efficacy of the antibiotic. This is the case for tetracyclines and macrolides (Table 6).
Antibiotic Treatment of Chlamydophila pneumoniae
| Antibiotic | Dose / Day |
|---|---|
| Azithormycin | 500 mg |
| Clarithromycin | 1000 mg |
| Telithromycin | 800 mg |
| Doxycycline | 400 mg |
| Gemifloxacin | 320 mg |
| Rifampicin (in combination with doxycycline or azithormycin) | 600 mg |
| Treatment duration for chronic course (no reliable data basis) | 2 - 3 months, if necessary 6 months for so-called reactive arthritis [108] |
The primary disease manifestation of Chlamydophila pneumoniae is pneumonia. The incidence is 1% and predominantly affects people older than 65 years [105,106]. The pneumonia is frequently accompanied by infections of the upper respiratory tract (pharyngitis, laryngitis, sinusitis). An initially slight disease expression, extrapulmonary manifestations [107] and a normal leukocyte count indicate atypical pneumonia, suggestive of Chlamydophila pneumoniae infection. Extrapulmonary manifestations include nervous system disorders including Guillain-Baré syndrome and meningoencephalitis, as well as reactive arthritis and myocarditis, which are significant with regard to the differential diagnosis of Lyme disease or Lyme neuroborreliosis.
The extrapulmonary manifestations frequently extend across a long period of time, i.e. through months or years. This also applies to the so-called reactive arthritis, whose differentiation from Lyme disease arthritis is sometimes difficult. Attention should also be paid to Guillain-Barré syndrome, which can last for months, and with a similar manifestation to that observed in Lyme disease. The association with a myocarditis is also similar, whereas meningoencephalitis occurs in the acute phase, almost simultaneously with pneumonia.
The chronic course of Chlamydophila pneumoniae infections is documented by several studies [108-114]. A relationship to Chlamydophila pneumoniae was also described in Alzheimer’s disease [115]. This finding is of importance with regard to chronic Lyme neuroborreliosis, for which the same associations have been demonstrated [116-119].
The laboratory diagnosis for Chlamydophila pneumoniae includes serology, the lymphocyte transformation test (LTT), and the detection of the microorganism using PCR.
The serological tests have severe inherent limitations. There is a considerable discrepancy between the serological findings, on the one hand, and pathogen detection using PCR, on the other hand [120,121]. A single test for IgG has a low sensitivity [122], whereas the sensitivity is quite good in cases of a definite increase in IgG between the acute phase and the further course of the disease. The diagnostic value of LTT for Chlamydophila pneumonia has not yet been validated in the literature. The chronic disease course obviously represents a chronic persistent infection. Chlamydophila pneumoniae could be detected both in the synovial fluid and in the liquor using PCR [108-110,113,114].
Antibiotic treatment of Chlamydophila pneumoniae is given in Table 6. The drug of choice is doxycycline; macrolides also exhibit good efficacy, particularly azithormycin; quinolones have a low efficacy [123], however, gemifloxacin has proven to be very effective [124].
Chlamydia trachomatis
The variable microbiological appearance of Chlamydia trachomatis is similar to that of Chlamyophila pneumoniae, described above. That the infectious form of Chlamydia trachomatis is present both intra- and extracellularly is decisive with regard to the efficient antibiotic treatment of the disease.
Chlamydia trachomatis is sexually transmitted and causes urogenital infection. The differential diagnostic problem with respect to Lyme disease results primarily from chronic arthritis, which is caused by persistent infection in both diseases. In Chlamydia trachomatis infection, this chronic arthritis is assigned to the so-called reactive arthritis even though that in some studies the pathogen was detected in the synovial fluid, which indicates persistent infectious arthritis [125,126]. Arthritis occurs in 1% of the cases with Chlamydia trachomatis urethritis. Reiter’s triad (arthritis, uveitis, urethritis) occurs in only 0.3% of the affected patients.
The disease can be easily detected by laboratory diagnostic tests in cases of existing urogenital infection. Nucleic acid amplification techniques (NAATs) for urethral smear or urine are available, which are also reliable for asymptomatic patients [127-129]. PCR has also a high sensitivity and specificity [130]. The diagnostic value of the serology and LTT has not yet been validated. Moreover, whether a chronic infection with Chlamydia trachomatis can be accompanied by seronegativity, as in Lyme disease, has not been clarified. Seropositivity can provide evidence of a previous infection, but does not allow conclusion with regard to a persistent infection with Chlamydia trachomatis. Theoretically, persistent or reproducible pathological LTT indicates prolonged infection, but scientific data for the diagnostic value of LTT are not yet available.
Treatment of Chlamydia trachomatis infection is similar to that of Clamydophila pneumoniae (Table 6). Duration of treatment is 3 months [108]. In case of arthritic Sulfasalazine [131] and treatment by TNF antibodies [132] were also proposed.
Yersinia enterocolitica Infection (Yersiniosis)
The infectious data and symptomatology of yersiniosis are compiled in Tables 1 and 7, respectively. Yersinia enterocolitica was already recognized as pathogen as early as the beginning of the 20th century. However, the true significance of the pathogen, in particular, under epidemiological aspects was first described in 1995 [133]. Acute illness due to Yersinia enterocolitica is subject to registration (according to German law). The pathogen penetrates into the intestinal wall and the mesenteric lymph nodes. Surface proteins and plasmid-bound virulence factors suppress immune system reactions of the host [134-137].
Yersiniosis (Y. enterocolitica) Symptomatology
| Stages | Symptoms |
|---|---|
| Early stage | Gradual development of gastroenteritis, pharyngitis, complications due to inflammation of the intestinal wall, mesenteric lymphadenopathy. |
| Excretory for months after abatement of gastroenteritis | |
|
|
|
| Late stage | Articular manifestations: Reactive arthritis in hip, knee, upper ankle, sacroiliac joints, arthralgias, ankylosing spondylitis, rheumatoid arthritis, sacroiliitis |
| Erythema nodosum, iridocyclitis, conjunctivitis, gastrointestinal complaints, abdominal pain, diarrhea, ulcerative colitis, nephritis, insulin-dependent diabetes mellitus, hepatitis (ANA positive, rheumatoid factor positive), myocarditis (rare), central and peripheral nervous system manifestations, multisystem disease | |
| Disease progression in stages with intervals of fewer complaints | |
| Correlation with thyroiditis | |
| Positive LTT | |
| Oscillating serological findings (correlation with disease expression) | |
The early stage of yersiniosis is essentially characterized by gradually developing gastroenteritis and pharyngitis and mesenteric lymphadenitis.
In contrast to other bacterial gastroenteritis, Yersinia enterocolitic gastroenteritis develops gradually and often becomes stressfull or perceivable only after a week [138-140]. Frequently, the infection is associated with pharyngitis because the pathogens remain in the lymphatic tissue of the tonsils and the pharyngeal wall, where they can also be detected by means of smear test. The concurrent occurrence of gastroenteritis with pharyngitis is typical for a yersiniosis [141].
The mean disease duration is approximately two to three weeks, but distinctly longer disease course have been described. The acute illness can be associated with numerous gastrointestinal complications, primarily as a consequence of a severe bacterial inflammation of the intestinal wall [142-144]. In addition, the disease can also affect many non-gastrointestinal organs [142,143,145-148]. The patients frequently remain excretory for months, even when the gastroenteritis has long since abated [139].
Yersiniosis can result in so-called reactive arthritis and is thus an important infectious disease in the differential diagnosis of Lyme disease. Since the disease can also sporadically occur [139] and frequently remains unrecognized, the anamnestic search for the typical manifestations of yersiniosis particularly symptoms of the early phase is of considerable importance.
Differentiation between Lyme disease and yersiniosis is made even more difficult by the fact that both infections can cause multisystem disease. The study by Saebo und Lassen [149], which describe the various disease manifestations in a retrospective study of 458 patients, is of particular importance for the depiction of chronic yersiniosis: chronic persistent arthralgias, ankylosing spondylitis, rheumatoid arthritis, iridocyclitis, chronic abdominal pains, chronic diarrhea, ulcerative colitis, nervous disorders, nephritis, thyroid disorders, insulin-dependent diabetes mellitus, chronic hepatitis, (multisystem diseases) and a substantial reduction of the overall life expectancy. Many of the different relationships were reported in further publications by these authors [150-155]. Studies suggesting a possible direct relationship between Yersinia and inflammatory intestinal disorders [155] close the pathophysiological circle between Yersinia, inflammatory intestinal disorders and enteropathic arthritides. Despite this, it should be noted that the relationship between Yersinia infection and the above-mentioned numerous disease manifestations (except for arthritis) have been inadequately analyzed. This may be due to the fact that the significance of “yersiniosis” as a disease has only been recently recognized.
In the differential diagnosis of yersiniosis with respect to Lyme disease and other co-infections, the importance is primarily based on the manifestation of a so-called reactive arthritis. This arthritis can last for months, and exhibit recurrent and symptom-free intervals in the disease course. The so-called reactive arthritis in yersiniosis can occur alone, but occasionally also in connection with conjunctivitis and urethritis, previously termed Reiter's syndrome [156]. As in Chlamydia infections and possibly in bartonellosis, the arthritis is probably the consequence of chronic persistent infection [157, 158].
The so-called reactive arthritis primarily affects the hip, knee and upper ankle joints as well as the sacroiliac joints; occasionally there are additionally chronic pains in the lumbosacral region [159].
In the differential diagnosis of Lyme disease, it is of particular interest that in some studies Yersinia enterocolitica was detected in articular effusions in case of the so-called reactive arthritis [157, 158]. Sometimes these arthritides last for many years. In addition, there is a relationship between yersiniosis and thyroiditis. All these (chronic arthritides, multisystem disorders, disease course lasting for years, correlation with regard to thyroiditis) can also be observed in Lyme disease.
The laboratory diagnostics of Yersinia enterocolitica infection is based on the seriological findings, on the LTT findings and on pathogen detection by PCR and culture.
As in Lyme disease, there is often seropositivity in asymptomatic patients. Information on a possible seronegativity in chronic yersiniosis is not available. In the disease course the serological findings can correlate with disease expression [159]. It is not rare that a highly significant pathological Yersinia LTT is found in patients whose complaints are primarily consistent with chronic Lyme disease. Positive yersinia LTT could be an indication of chronic persistent infection especially when reproducible.
Pathogen detection is possible, particularly in articular effusion and in lymphatic tissue of the intestine and in early stages of the disease by throat swab. Data on the sensitivity of pathogen detection using PCR or culture methods does not exist in the relevant literature. In Yersinia-PCR-positive patients, the serology was positive in 70% and LTT in 50% of the cases analyzed [160]. In the initial detection of Yersinia enterocolitica using culture methods, IgA and IgG bands were found in immunoblot assay in patients experiencing a chronic course of the disease. The continuous detection of IgA antibodies was obviously an expression of persistent infection; in this context the pathogens were detected in the intestinal mucosa and in lymphatic tissue. Hence, this was a definitely chronic, persistent Yersinia enterocolitica infection [161]. The antibiotic treatment of Yersinia enterocolitica infection is presented in Table 8.
Antibiotic Treatment of Yersinia enterocolitica Infection
| Antibiotic | Dose / Day |
|---|---|
| Ceftriaxone + Gentamycin | 2 g + 240 mg |
| Ciprofloxacin | 1000 mg |
| Trimethoprim and sulfamethoxazole | 875 and 125 mg, x 2 daily |
| Gentamycin Doxycycline |
240 mg 400 mg |
| Piperacillin Entapeneum |
8 g 1g |
| Rifampicin (in combination with doxycycline or azithormycin) | 600 mg |
| Treatment duration for chronic course (no reliable data basis) | 2 - 3 months, if necessary 6 months for so-called reactive arthritis [108] |
Yersiniosis frequently abates within a few weeks so that an antibiotic treatment is not generally recommended. This also applies with regard to excretors. Antibiotics are used only in cases with severe disease courses, in particular with sepsis. Y. enterocolitica produces beta-lactamases with the consequence that penicillin, ampicillin and cephalosporins of the first generation are ineffective [162,163]. Resistance to macrolides is also frequent. It is disputed whether early antibiotic treatment (i.e. for gastroenteritis) prevents reactive arthritis [164].
The differential diagnosis of chronic yersiniosis vs chronic Lyme disease, is thus extremely difficult because of the overlap of various clinical symptoms. In cases where both infections are present in their chronic form, a differentiation is often impossible.
Mycoplasma pneumoniae Infection
The differential diagnosis between Lyme disease und Mycoplasma pneumoniae infection or the recognition of the co-infection by Mycoplasma pneumoniae is problematical because both diseases exhibit similar manifestations; this applies to the extrapulmonary manifestations of Mycoplasma pneumoniae infection: disorders of the CNS, musculoskeletal system, heart, kidney and eye.
Data related to Mycoplasma pneumonia infection are given in Table 1 and 9. In the foreground is the atypical pneumonia, frequently linked to symptoms of the upper respiratory tract. There are no data available in the literature with respect to the frequency of extrapulmonary manifestations.
Mycoplasma pneumoniae Infection: Symptoms
| Pulmonary
and related (older
people in nursing and old people’s homes are frequently affected) Incubation period 3 weeks |
Atypical pneumonia (3% - 10% of cases.) bronchitis, pharyngitis, rhinitis, earaches, sinusitis |
| Extrapulmonary |
Maculopapular exanthema,vesicular dermatitis CNS disorders (are rare): encephalitis, meningitis, myelitis, cranial neuropathy, cerebellar ataxia |
| Gastrointestinal | Hepatitis, pancreatitis |
| Rheumatic | Arthritis: arthralgias, myalgias, polyarthritis |
| Cardiac | Cardiac arrhythmias, atrioventricular block, myocarditis |
| Glomerulonephritis | |
| Uveitis |
Mycoplasma pneumoniae is considered to be the most important pathogen of atypical pneumonia. However, pneumonia only occurs in approximately 3% - 10% of the cases in Mycoplasma pneumoniae infection [165]. In most cases, the infection results in a banal bronchitis [165], pharyngitis, rhinitis, earaches, and sinusitis [163].
All the extrapulmonary disease manifestations listed in Table 9 are seldom [166-174]. In patients with arthritis, Mycoplasma pneumoniae was detected in the synovial fluid by PCR [171], which is an indication of a direct relationship to infection.
The detection of pathogen in articular effusion and many extrapulmonary manifestations of the disease document the chronic course of the disease in cases with Mycoplasma pneumoniae infection. However, precise data on the chronic course of the disease are not available in the literature. In particular, whether a chronic infection, especially with extrapulmonary disease manifestation, can persist with seronegativity is unclear. Seropositivity documents infection, but cannot serve as a proof for chronic persistent Mycoplasma pneumoniae infection.
The literature on the relationship between Mycoplasma pneumoniae infection and neurological disease manifestations is extensive. The publications primarily refer to neurological complications in pneumonia, i.e. the early phase of Mycoplasma pneumoniae infection. Neurological manifestations involve both the early phase, i.e. the point in time of existing pneumonia due to Mycoplasma pneumoniae, and later disease stages. Changes in the region of the brain stem [175, 176], myelitis [177-187], Guillain-Barré syndrome [188-193], encephalitis [185,187,194-200], meningitis [194], polyradiculopathy [178], peripheral facial paresis [201, 202], optical neuritis and hemorrhagic leukoencephalitis [190], peripheral polyneuropathy[194], cranial nerve neuritis [192], radiculitis [192] have been described.
The frequency of neurological symptoms in connection with Mycoplasma pneumoniae varies between 1‰ [203], 1% [204], and 5% [205]. The pathogen has been repeatedly detected by means of culture methods or PCR [182,193,194].
The detection of pathogen in the serum and liquor is considered to be proof that the neurological manifestations are mediated by direct infection and not by immunological responses [193]. However, the connection between Mycoplasma pneumoniae and neurological manifestations is not undisputed [203, 206].
Other extrapulmonary manifestations mentioned in the literature include hepatitis, hemolytic anemia, Schönlein-Henoch purpura, disorders of the muscular-skeletal system, of the skin and other organs [179], macula edema[194], bilateral uveitis [207], nephritis [208], arthritis, hepatitis and pericarditis [208].
The laboratory diagnostics for Mycoplasma pneumoniae include the serology, which as in most infectious diseases becomes positive after several weeks. Seroconversion is therefore significant for the chronic disease course. Seropositivity substantiates the infection, but not the disease. Whether a chronic infection can also exist in seronegative cases has not yet been scientifically clarified. The LTT for Mycoplasma pneumoniae has not yet been validated.
Detection of the pathogen, e.g. in articular effusion by PCR and culture is possible but difficult and has a low sensitivity. Consequently it is not part of the routine diagnostic procedures.
In the antibiotic treatment of Mycoplasma pneumoniae the drugs of choice are azithormycin 500 mg/day [209], levofloxacin 500 mg/day [210] and doxycycline 400 mg/day.
Human Granulocytic Anaplasmosis (HGA)
The pathological importance of human monocytic ehrlichiosis (HME) and human granulocytic anaplasmosis (HGA), which is also called human granulocytic ehrlichiosis (HGE), was discovered in 1986 and 1994, respectively [211, 212]. The two infectious diseases resemble each other clinically and with regard to the laboratory findings. The pathogens develop in monocytes (HME) or in granulocytic leukocytes (HGA). Thus, their localization is exclusively intracellular.
The pathogens are transmitted by infected ticks, primarily via Ixodes scapularis in the United States and via Ixodes ricinus in Europe. The reservoirs are the white-footed mouse for HGA and the red deer and human beings for HME (Table 1). Other modes of transmission are also discussed, including mother-child transmission, blood transfusions, direct contact with infected animals and transmission form person to person [213-219].
In connection with ehrlichiosis or anaplasmosis, two pathogens are to be noted, respectively, namely Ehrlichia chaffeensis [220] and Anaplasma phagocytophilum [221]. E. chaffeensis infects monocytes and Anaplasma Phagocytophila granulocytes.
Ehrlichia chaffeensis is the pathogen of human monocytic ehrlichiosis (HME), a rare infectious disease, which occurs primarily in the USA and in some regions of South America, but practically nowhere else on earth. Ehrlichia phagocytophila is the pathogen of human granulocytic anaplasmosis (HGA), another extremely rare disease in the USA with an annual incidence of approximately 10 / 1 million inhabitants [222]. HGA, is an important co-infection of Lyme disease in the USA, but not in Europe.
The pathogen can be simultaneously transmitted with Borrelia burgdorferi with the consequence of a double infection. HGA exhibits many symptoms, which also occur in Lyme disease.
Scientific reports on illnesses due to HGA in Europe are rarities [223]. However, studies in Northern Italy showed that 24% of the ticks (I. ricinus) were infected by Ehrlichia chaffeensis or Anaplasma phagocytophilum. Similar findings have been substantiated in the Netherlands and in Poland, whereas in Germany the frequency is approximately 2% [224-229]. In the East Coast of the United States it is much higher and reaches approximately 30% - 40% [230,231].
In Europe, in patients with Lyme disease the seroprevalence for Anaplasma phagocytophilum is approximately 10% [232-234]. Similar findings were also obtained in the USA [235]. Since seroprevalence merely expresses the frequency of the infection, but not an ungoing active infection of HGA, no reliable statements about the prevalence of the disease can be made. According to the laws of probability, a concurrent HGA infection in patients with Lyme disease might amount to a few percent at most. There are no data available in the literature on chronic HGA courses. However, subacute and chronic courses are discussed [236, 237].
The incubation period, i.e. the time between tick bite and emergence of acute illness, is approximately one week [238]. In the acute phase clusters of bacteria are located intercellularly (morale) [239].
The clinical symptoms comprise fever, influenza-like symptoms, headache, joint and muscle pains, coughing, CNS disorders, which include meningitis. Such changes occur frequently and are - particularly in cases involving a febrile clinical picture with the above-mentioned symptoms - an indication for HGA. Indications of HGA are the pathological laboratory findings of leukopenia, thrombocytopenia, and elevated transaminases. The pathogen is localized intracellularly. Anemia and elevated creatinine can rarely occur.
The diagnosis of HGA (also as co-infection) is based on the anamnesis, on the clinical symptoms and laboratory findings, including the serological findings [240-243] and the detection of pathogens. The specific antibodies appear two to three weeks after disease onset and persist following the decline of the disease. Therefore, a positive serology does not confirm ongoing infection of HGA. The pathogen is detected in blood smear, which is not often successful; [244-247], or by PCR from whole blood. The detection by PCR was successful in 20% - 80% of the cases analyzed, with a sensitivity of 60% - 80% [248-251].
Doxycycline is the recommended therapy, also for children. Precise literature with regard to an adequate treatment is not available.
BABESIOSIS
Babesiosis, similarly to HGA, is an important co-infection in the USA, but not in Europe. Babesiae are protozoa and result in lysis subsequent to invasion of erythrocytes. Two species of Babesia are pathogenetically significant, namely Babesia microti and Babesia divergens [29,30]. Babesia microti is the predominant pathogen in the USA and Babesia divergens in Europe [cf. 252]. The transmission of the pathogen occurs primarily via ticks, namely Ixodes ricinus in Europe and Ixodes scapularis in the USA [29,30]. Babesia microti has been found as a co-infection in Lyme disease [253-255]. Other modes of transmission are blood transfusions [31] and perinatal infection [32,33]. The reservoir is the cattle and probably other vertebrates (Table 1).
Since 1956 a total of only 30 cases has been reported in Europe. The majority of these patients were splenectomized. The prevalence of Babesia microti and Babesia divergens in ticks is 10% - 20% in Europe [256-258], and somewhat higher in the U.S.A. [259]. The seroprevalence with regard to Babesia microti and Babesia divergens is 0% in European patients with Lyme disease [260,261] and thus is in stark contrast with the frequency of the pathogen in ticks.
The situation in the USA is different, where the seroprevalence is approximately 10% - 20% [262-265] where the disease was reported to occur more frequently, in some cases with severe disease courses [266-269]. This difference can obviously be only explained by the fact that Babesia microti, the predominant pathogen in the USA, has a much higher virulence than Babesia divergens. Thus, Babesiosis dos not play a major role in Europe unless the patient contracted the disease in a foreign country, e.g. in the USA.
The clinical picture presents as a febrile, influenza-like medical condition with chills, fever, arthralgias, myalgias, and gastrointestinal symptoms. Severe disease courses only occur in non-immunocompetent patients. The diagnosis is based in addition to the anamnesis and the clinical picture, on the serological findings [270,271] and on the detection of the organism. There is a poor correlation between serologic titer and symptomatology [271]. Detection of the pathogen in blood smear is difficult and frequently requires repeated examinations. The sensitivity of the PCR to detect the organism is higher [272].
Treatment is carried out with atovaquone, azithormycin, and clindamycin, if necessary in combination with quinine.
Following assessment of all data available, it seems that babesiosis - due to the dominant European pathogen, Babesia divergens - does not represent a major health hazard and thus is of little consequence as a co-infection in Lyme disease.
RICKETTSIOSES
Attention should be directed to the fact that the causative agent of bartonellosis also belongs to the Rickettsia family. In the USA the most important and more frequent rickettsiosis is the Rocky Mountain Spotted Fever (RMSF), a potentially fatal, but normally curable disease. The clinical picture is primarily characterized by high fever, pronounced malaise, abdominal complaints, and a generalized exanthema. Occasionally, the disease is also linked with CNS manifestations comprising focal neurological deficits and cerebral seizures.
Various different rickettsioses caused by different Rickettsia subspecies are distinguished worldwide. The transmission generally occurs via ticks, but also via mites, fleas, and lice (Table 1). Generalized exanthema - the so-called localized eschar (black wound) - as well as fever, headaches and severe muscle pains are typical symptoms of the disease.
The most important rickettsiosis in Europe is the Mediterranean spotted fever caused by Rickettsia conorii. The disease primarily affects Southern Europe. Chronic courses have not been described in the literature. In the early stage, it does not represent differential diagnostic difficulty with respect to Lyme disease, because of the endemic conditions and the presence of the typical exanthema. Treatment is performed with doxycycline.
TULAREMIA
Tularemia is caused by the pathogen Francisella tularensis. Transmission occurs via mosquitoes. The disease reservoir comprises many vertebrates.
The main disease manifestations are fever, headaches, malaise, swollen lymph nodes, pharyngitis, eschar (black wound), emesis, pneumonia and the erythematosus papularulcerative lesion at the site of the black spot bite (central eschar, “tache noire”).
Relapses can occur, but persistent chronic courses have not been described in the relevant literature. Differential diagnostic problem can occasionally occur with regard to early lyme decrease in cases lacking to crythema migrants.
The treatments of choice are tetracyclines and ciprofloxacin. Betalactamases are inefficient.
Q FEVER
In contrast to rickettsioses and tularemia, Q fever can exhibit a chronic course. However, There are no significant differential diagnostic problems with regard to Lyme disease. Q fever normally is endemic and as a rule is caused by contact with livestock via inhalation or oral transmission of the pathogen, or by person to person contact. Admittedly, Coxiella burnetii can also be found in other reservoirs, e.g. in ticks (Table 1), but two items are decisive for the diagnosis: the endemic occurrence and the contact with (diseased) livestock and their products (e.g. milk products) and other infected patients.
The decisive diagnosis with regard to a possible Q fever is thus the occupational activity or the contact with agriculture and livestock. In sporadic cases the frequent consumption of raw milk or the contact with diseased cattle (abort) can be indication of an infection hazard.
Significant disease manifestations include transitory influenza-like clinical picture, pneumonia and hepatitis. Other manifestations comprise erythema, pericarditis and/or myocarditis, meningitis, encephalitis [273-275] and myelitis [276, 277].
Q fever can persist for months or years indicative of a chronic course. Chronic endocarditis is the dominant disease manifestation but pericarditis and Guillain-Barré syndrome also occur [278]. The diagnosis is verified by serological tests detecting the presence of specific antibodies.
HUMAN PARVOVIRUS B19 INFECTION
Human parvovirus B19 infection is transmitted by respiratory tract infection (droplet infection) or during pregnancy and blood transfusion (Table 1). Its reservoir is the human being. The disease can exhibit a chronic course of months or years [279]. Whether parvovirus B19 causes chronic myocarditis and cardiomyopathy is a matter of dispute [280-283]. The chronic course is verified by the detection of pathogen in articular effusions, in the myocardium, bone marrow, and blood [279-285]. With regard to Lyme disease, the following differential diagnostic disease manifestations are relevant: persistent or recurrent arthropathy, myocarditis and cardiomyopathy. Fifth disease or erythema infectiosum is a typical skin manifestation of parvovirus B19 infection in children, but does not normally occur in adults. Arthralgias can last for months or years.
Campylobacter jejuni
Campylobacter jejuni is a small Gram-negative bacterium whose pathological significance was recognized around 1980. Campylobacter jejuni is among the most frequent pathogens worldwide, causing acute diarrhea. Sources of infection are game and domestic animals, especially poultry, various animal products and contaminated water [286]. The pathogen can persist in a coccoid form, but also in its normal form, for months in unfavorable conditions. It penetrates epithelial cells of the intestine causing their destruction, possibly by means of toxins [287, 288].
The main clinical manifestations of Campylobacter jejuni infection are gastroenteritis and abdominal complications in the early phase and reactive arthritis and Guillain-Barré syndrome in the late phase of the disease.
Campylobacter jejuni has differential diagnostic significance due to the late manifestations of the disease, namely due to reactive arthritis and Guillain-Barré syndrome.
Reactive arthritis in Campylobacter jejuni infections is seldom with a frequency of about 2.6% [289-292]. In connection with a Campylobacter jejuni infection, Guillain-Barré syndrome has an unfavorable prognosis [293]. Its incidence is approximately 1‰ [294]. Reactive arthritis occurs approximately one to two weeks after gastroenteritis [288] and Guillain-Barré syndrome approximately two months after the onset of infection [295].
Antibiotic treatment reduces the duration of gastroenteritis. The drugs of choice are erythromycin (1500 mg/daily), azithormycin (500 mg daily) and ciprofloxacin (1000 mg daily). Macrolides [296] and quinolones are primarily recommended, but resistance to them can occur [297]. Resistance to trimethoprim und beta-lactamases also exists [298].
Reactive arthritis
The term “reactive arthritis” characterizes arthritides, which bear a relationship to certain infectious diseases. In former times the term “Reiter syndrome” was used in cases of concurrent infection of the urethra and the uvea. Arthritis, urethritis and uveitis were termed the Reiter’s triad [299,300]. Pathogens, which can induce reactive arthritis include Chlamydia trachomatis, Chlamydia pneumoniae, Yersinia enterocolitica, Salmonellae, Shigella, Campylobacter (usually Campylobacter jejuni), Mycoplasma pneumoniae and possibly Clostridium difficile.
The term “reactive arthritis” is not a defined disease (nosological entity), but rather a concept for the classification of disease relationships with respect to the pathophysiology. The term “reactive arthritis” is problematical. In various infections with reactive arthritis, the pathogens were detected in the synovia and joint fluid. This is true for Chlamydophila pneumoniae [108-110], Chlamydia trachomatis [125,126] and for Yersinia enterocolitica [157,158]. The pathogen was also found in the synovia in cases with arthritis in connection with Mycoplasma pneumoniae [171].
Reactive arthritis occurs days to weeks after the onset of infection. It predominantly affects the joints of the lower extremities. In cases with disease duration of less than 6 months, the term “acute reactive arthritis” was designated and for those with disease duration of more than 6 months, the term “chronic reactive arthritis" is used.
In 50% of the cases, the joints of the upper extremities are also affected, including the small joints, and the arthritis can be accompanied by tendonitis (enthesitis) [301-304].
One of the most important differential diagnoses of reactive arthritis is Lyme arthritis (chronic Lyme disease, Lyme disease in the late stage, stage III).
Reactive arthritis can be associated with other disease manifestations. These extra-articular symptoms in cases of reactive arthritis include urogenital symptoms, conjunctivitis, uveitis, aphthae, hyperkeratotic skin lesions in the region of the sole of the foot and the palm of the hand, nail changes as in psoriasis and genital lesions, such as balanitis.
In the diagnosis of a “reactive arthritis” anamnestic research is to be performed to determine whether there are indications of one of the above-mentioned infections. Accordingly, the following significant anamnestic considerations are important: Chlamydia trachomatis infection with and without symptoms, enteritis and atypical pneumonia.
Additionally, it should be remembered that arthritides are also found in Chlamydophila pneumoniae und Mycoplasma pneumonia infections and that in Chlamydioses, Yersiniosis and also in cases of Mycoplasma pneumoniae, the pathogen has been detected in the synovial membrane or in the articular effusions, respectively.
Using laboratory diagnostic tests (culture, serology), an existing or previous infection can be detected in 50% of the cases. Other laboratory tests, particularly inflammation markers, like the erythrocyte sedimentation rate (ESR), the C reactive protein (CRP) and leukocytosis are not relevant in cases of reactive arthritis. With regard to Chlamydia trachomatis, pathogen detection in a urethral smear or in urine using PCR or NAATs is appropriate.
Chronic courses, i.e. a duration of illness exceeding 6 months, are observed in nearly 20% of the patients [305].
NSAIDs are used for treatment, but only for pain relief since they have no influence on the disease course or disease duration. In contrast, sulfasalazines [131] and TNF antibodies have certain efficacy. Antibiotic treatment is recommended in cases of acute chlamydiosis with the objective of reducing the frequency of reactive arthritis. However, studies in this direction are needed [306]. In cases of chronic reactive arthritis, the efficiency of antibiotic treatment is a matter of controversy [304,307-312].
SUMMARY OF THE CLINICAL SYMPTOMS AND THE TREATMENT OF LYME DISEASE AND CHRONIC CO-INFECTIONS
An informative overview of the different disease manifestations of Lyme disease and the significant co-infections is given in Table 10. The overview shows than there is substantial overlap in symptoms in cases of Lyme disease, bartonellosis, Yersinia enterocolitica and Mycoplasma pneumonia infections. Additionally, Chlamydophila pneumoniae also exhibits some overlap in the symptomatology with Lyme disease. Chlamydia trachomatis and Campylobacter jejuni are primarily characterized by reactive arthritis and rarely by Guillain-Barré syndrome. Only in cases with chronic Lyme disease does the antibiotic treatment (Table 11) involve the use of cephalosporins of the 3rd generation and, if necessary, of carbapenems. Otherwise, the focus is generally on tetracyclines, macrolides, to some extent on quinolones, particularly gemifloxacin, all of which, exhibit an intracellular and extracellular efficacy.
Disease Manifestations of Chronic Lyme Disease and Chronic Co-infections (Overview)
| Disease | Symptomatology | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| GenS | MuSk | NS | Skin | Heart | Eye | GI | UG | rA | GBS | LA | |
| Lyme disease | + | + | + | + | + | + | + | + | (+)* | + | + |
| Bartonellosis | + | + | + | + | + | + | + | + | + | + | + |
| Y. enterocolitica | (+) | + | + | + | + | + | + | + | + | ||
| M. pneumoniae | (+) | + | + | + | + | + | + | + | + | + | |
| C. pneumoniae | + | + | + | + | |||||||
| C. trachomatis | + | + | + | ||||||||
| C. jejuni | + | + | |||||||||
Y. enterocolitica = Yersinia enterocolitica; M. pneumoniae = Mycoplasma pneumoniae; C. pneumoniae = Chlamydophila pneumoniae; C. trachomatis = Chlamydia trachomatis, C. jejuni = Campylobacter jejuni; GenS = general symptoms (fatigue, head aches, lassitude); MuSk = musculoskeletal symptoms (arthritis, arthralgias, myalgias); NS = symptoms of the nervous system (CNS, polyneuropathy, radiculopathy); Skin = skin lesions (erythema migrans, ACA in cases of Lyme disease e.g. infected skin injury); LA = lymphadenopathy; Heart = heart disease (myocarditis, cardiomyopathy, pericarditis); Eye = eye disease (uveitis, conjunctivitis, optic neuritis); GI = gastrointestinal complaints; UG = urogenital symptoms; rA = reactive arthritis; GBS = Guillain-Baré syndrome; + = positive; (+) = presumption based on general symptoms in cases of yersiniosis and Mycoplasma pneumoniae infection;+
* = probably chronic infectious, hypothetical autoimmune origin (mimicry).
Antibiotic Treatment of Chronic Lyme Disease and Chronic Co-infections
Ceph3 = 3rd generation cephalosporins; Carbap = carbapenems; Tetracyc = Tetracyclines; Macrol = macrolides; Quinol = quinolones; TMSU = trimethoprim and sulfamethoxazole; Rifa = rifampicin. *Gemifloxacin
* subsequent to testing: Piperacillin
** Ceph3 (if necessary + gentamycin)
*** Erythromycin
CONCLUSION
At the end of the 20th century, a number of infectious diseases attracted the attention of medical and health policy interests. This is primarily due to the fact these diseases frequently have a chronic course. In Europe and North America, but also in many other areas of the world, these chronic diseases are caused by the following pathogens: Borrelia burgdorferi, Bartonella henselae, Mycoplasma pneumoniae, Chlamydophila pneumoniae, Chlamydia trachomatis, Yersinia enterocolitica. In North America, HGA (Human Granulocytic Anaplasma) and Babesias are also important, whereas infections with these pathogens are a rarity in Europe. In addition, other pathogens discussed in the text are of secondary importance with regard to their frequency of occurrence. Borrelia burgdorferi is transmitted by ticks; in some cases this also applies to Bartonella henselae. The two diseases can also be simultaneously transmitted by tick bites. The remaining pathogens mentioned have other modes of transmission. — Of all the above-mentioned infectious diseases, Lyme borreliosis (Borrelia burgdorferi) is by far the most frequent infectious disease with a chronic course. In addition, this disease (Lyme borreliosis, Lyme disease) is the most thoroughly investigated of all the mentioned infectious diseases. On the basis of the special status of Lyme borreliosis, the other infections have been termed “coinfections” in the literature. Lyme borreliosis can be accompanied by one or more coinfections (double or multiple infections). Coinfections exacerbate the expression of the disease and hinder the therapeutic success. The symptomatology of Lyme borreliosis and the so-called coinfections exhibit high degrees of overlap. A subtle diagnostic analysis is required to account for all of the infectious diseases, which could (possibly) be present. The diagnostic and therapeutic options in cases of chronic infectious diseases are limited. This applies to Lyme borreliosis and even more for the coinfections. No adequate laboratory methods are available to the important Bartonella henselae,coinfection and there are no official guidelines available with regard to its antibiotic treatment. All of the above-mentioned pathogens are capable of intracellular localization; thus, (with the exception of Borrelia burgdorferi) only intracellularly acting antibiotics are used. Despite this, the failure rate of the antibiotic treatment of the coinfections is high; on the other hand, with particular regard to Bartonella henselae the opinions on an adequate antibiotic therapy are very controversial. Since the clinical and scientific significance of these chronic infectious diseases has meanwhile been recognized, it is now imperative to develop and improve diagnostic and particularly therapeutic measures for the chronic infectious diseases.


