DJ-1 Mutations are Rare in a Swedish Parkinson Cohort
Abstract
Mutations in the PARK7 gene, DJ-1, have been reported to cause early-onset and familial Parkinson’s disease (PD). The function of DJ-1 and how it contributes to the development of the disease is not clear today, but several studies report that DJ-1 is responsive to oxidative stress and important for the maintenance of mitochondria. We have screened three coding regions of DJ-1 (exon 2, 5 and 7) in a Swedish Parkinson cohort. The Swedish PD material consisted of 67 patients with a self reported positive family history of PD and 77 patients with early-onset of disease (≤50 years old). We detected two patients with the previously reported synonymous mutation, Ala167Ala (c.501A>G, rs71653621), in exon 7. No Ala167Ala carriers were identified among 213 neurologically healthy Swedish controls. Mechanisms by which the synonymous Ala167Ala mutation can have consequences are unknown. It may affect the mRNA stability, secondary structure of mRNA, synthesis, turnover, protein folding and function. We could show a 1.3% decrease in DJ-1 mRNA folding energy in the A<G substituted sequence compared to the wild type sequence in silico, suggesting a possible small effect of Ala167Ala on DJ-1 gene function. This is the first report on an identified DJ-1 mutation in Swedish PD patients. Our results, in combination with those of previous studies, strengthen the hypothesis that alterations in DJ-1 are not a common cause of familial and early-onset PD world-wide.
INTRODUCTION
Parkinson’s disease (PD) is a neurodegenerative disorder caused mainly by progressive loss of dopamine neurons in substantia nigra pars compacta. Pathways proposed to trigger onset of PD include oxidative stress and dysfunction of mitochondria [1]. The PARK7 gene, DJ-1, has been reported to be responsive to oxidative stress and important for the maintenance of mitochondria [2,3]. DJ-1 was originally identified and described as an oncogene [4] and the 189 amino acid protein is highly conserved across species [5]. The DJ-1 protein is abundantly expressed throughout the body and in the normal human brain it is moderately expressed in neurons and in astrocytes throughout the CNS [6,7]. Oxidative modifications of DJ-1 have been reported in brain tissue from patients with sporadic PD and Alzheimer’s disease compared to controls [8]. It has been suggested that DJ-1 protect neurons against oxidative stress by acting as a redox-dependent chaperone [9]. The protein is normally found in the cytoplasm and oxidative stress promotes relocation to mitochondria where it protects against mitochondrial toxins [2,10]. DJ-1 can bind RNA in an oxidative-dependent manner and the DJ-1/RNA complex dissociates after oxidative stress [11,12]. Structural studies of DJ-1 have suggested oxidation of Cysteine at residue 106 to be essential for DJ-1 to exert its full activities [3]. However it remains to be shown how oxidation of Cys106 leads to antioxidative and/or cytoprotective effects through conformational changes and how PD associated DJ-1 mutations affect these processes.
In 2001 the PARK7 locus on chromosome 1p36 was linked to a family with autosomal recessive early-onset parkinsonism (AREP) in the Netherlands [13]. The following year the same locus was linked to a family with AREP in Italy [14]. Two mutations in DJ-1 were later reported to cosegregate with disease, a 14kb deletion in the Dutch PD family and a Leu166Pro substitution in the Italian PD family, and both lead to loss of gene function [5]. The DJ-1 mutations are recessively inherited and result in PD with early-onset, overall mutations identified to date are rare, causing ~1% of the PD cases world-wide and no mutations have been reported in Swedish PD cases [15]. In the Caucasian PD population the majority of DJ-1 mutations leading to changes in the amino acid sequence have been found in exons 2, 5 and 7 [16]. We therefore sequenced these exons in a Swedish Parkinson cohort to search for known and new variable sites.
MATERIAL AND METHODS
Subjects
Exons 2, 5 and 7 and the exon/intron borders of DJ-1 were sequenced in a Swedish PD material consisting of 144 individuals (mean age 64.3 years, 56.9% men). 77 of the PD patients had an early disease onset (≤50 years old) and 67 of the patients had a self reported positive family history of PD in one or more first-, second- or third-degree relatives (15 of these patients had early-onset as well). 66 of the early-onset cases have previously been investigated for possible changes in copy numbers in exon 1, 3, 5 and 7 of DJ-1 although no aberrations were detected [17]. These patients were screened for Parkin point mutations and Parkin, α-Synuclein, UCH-L1 and PINK1 exon copy number aberration as well. No known PD causing Parkin mutation or copy number aberration was found. Control subjects consisted of 213 neurologically healthy participants from the Stockholm area (mean age 75.8 years, 41.3% men). The material was obtained after informed oral and written consent and approval of the local ethics committee at Karolinska Institutet and University of Gothenburg. All PD subjects met the United Kingdom Parkinson's Disease Society Brain Bank Criteria for PD except that more than one affected relative was allowed [18]. DNA was extracted from blood according to standard protocol.
Sequencing
Samples from PD patients were analyzed with automated capillary sequencing. Polymerase chain reaction (PCR) was carried out using Taq DNA polymerase and the following primer sequences: exon 2 forward primer 5´-TCTCAGGG TTGCAATGAAAG-3´; reverse primer 5´-AAGCGTTAA ATGTGAGCAGTG-3´, exon 5 forward primer 5´-AAATAGGTCAGAGAGCTTGTGG-3´; reverse primer 5´-TCAAACCATCGAATGAAAGG-3´ and exon 7 forward primer 5´-ACAGTGTTGGGTTTATATGCTG-3´; reverse primer 5´-GGACAGCGACTTCTGAACAC-3´. 40 cycles were run at 95°C for 45 s, 56°C for 40 s and 72°C for 1 min followed by 7 min terminal elongation at 72°C. The PCR products were 337 bp (exon 2), 258 bp (exon 5) and 378 bp (exon 7) of the DJ-1 gene. Purification of the amplified fragment was performed by using QIAquick® PCR Purification kit (Qiagen, Hilden, Germany). The isolated DNA fragments were sequenced using a DTCS kit followed by automated capillary gel electrophoresis (CEQ 2000 system, Beckman Coulter Inc., Fullerton, CA, USA), following the provider’s instruction.
Pyrosequencing
To specifically screen neurologically healthy controls for the Ala167Ala mutation in exon 7 we used pyrosequencing [19] and the following primer sequences; forward primer 5´-AGACGGCCTGATTCTTACAAGC-3´; reverse primer 5´-GGCCTGTTTCTCTAAGTGATCG-3´ and sequencing primer 5´-CTTCGAGTTTGCGC-3´. The reverse primer was biotinylated at the 5´-end. Positive controls for the Ala167Ala mutation were used in the pyrosequencing assay to guarantee accuracy of the method as well as water controls. PCR was carried out with Taq DNA polymerase to amplify a 175 bp fragment. 45 cycles were run at 95°C for 20 s, 57°C for 20 s and 72°C for 30 s followed by 7 min terminal elongation at 72°C, after which the biotinylated PCR product was immobilized on streptavidin-coated beads by mixing at 2500 rpm for 10 min at room temperature according to manufacturer’s instructions. The immobilized DNA template was then captured onto filter probes (PyroMark Vacuum Prep Tool, Biotage AB, Uppsala, Sweden). The filter probes were flushed with 70% ethanol, denaturation solution, washing buffer and the single-stranded template was annealed to a reverse sequencing primer at 80°C for 2 min followed by cooling to room temperature. All solutions used in sample preparation were prepared according to manufacturer’s instructions (Biotage AB, Uppsala, Sweden). Samples were analyzed on an automated pyrosequencer using a PSQ 96 System together with single SNP Software and SNP Reagent Kits (Biotage AB, Uppsala, Sweden).
Prediction of mRNA Secondary Structure
To evaluate the possible effect of the identified Ala167Ala mutation on the DJ-1 mRNA level, the secondary structure was predicted using the publicly available online software mfold version 3.2 [20,21]. Partial DJ-1 mRNA sequences of 141 nucleotides including flanking sequences (70 nucleotides) on either side of the mutation were analyzed and compared to the wild type sequence.
RESULTS
In 144 PD cases we identified the synonymous mutation, Ala167Ala (c.501A>G, rs71653621), in exon 7 of DJ-1 in two PD patients (Fig. 1 and Table 1). No Ala167Ala carriers were identified among the 213 neurologically healthy Swedish controls. To test whether the synonymous Ala167Ala substitution has a possible effect on the secondary structure of DJ-1 mRNA we performed a structure analysis in silico. Our results indicated a small decrease in mRNA folding energy (1.3%) in the A>G substituted sequence compared to the wild type sequence (Table 2).
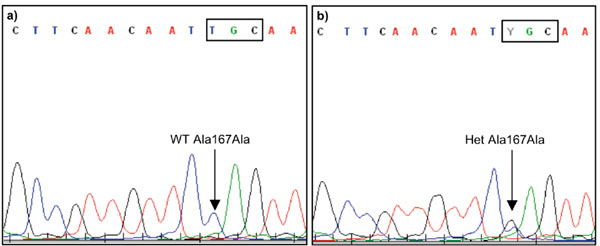
Results from automated capillary sequencing of exon 7 in DJ-1 including the Ala167Ala (c.501A>G, rs71653621) mutation using a reverse sequencing primer (T>C). a) A homozygous wild type (WT) T/T, Ala167Ala carrier and b) a heterozygous (Het) T/C, Ala167Ala carrier.
Patient Information on the Two Individuals with Parkinson’s Disease (PD) Heterozygous for the DJ-1 Mutation Ala167Ala (c.501A>G, rs71653621) in Exon 7
| Sex | Family history | Age at sampling | Age of onset | Brief history | |
|---|---|---|---|---|---|
| CASE 1 | Female | Brother With PD | 71 | 69 | Moderately advanced PD, ON/OFF with some motor fluctuations but dominating non-motor symptoms in off. |
| CASE 2 | Male | No known heredity | 65 | 48 | Benign course. Minimal motor fluctuations but walking unassisted after 24 years disease. Diagnosed with Alzheimer’s disease as well at age 66. |
In Silico mRNA Folding Energy (kcal/mol) for the Wild Type (WT) and Mutated (MUT) Ala167Ala DJ-1 Sequences
| Individual structures | Minimum free energy (kcal/mol) | |
|---|---|---|
| WT Ala167Ala | MUT Ala167Ala | |
| Structure 1 | 39.10 | 38.80 |
| Structure 2 | 38.90 | 38.60 |
| Structure 3 | 38.00 | 37.70 |
| Structure 4 | 37.30 | 37.00 |
| Structure 5 | - | 37.00 |
| Average | 38.33 | 37.82 |
DISCUSSION
The number of DJ-1 mutations described today is low and they account for only a few PD cases in different populations [16]. In the present study, we have searched for mutations in exon 2, 5 and 7 and in the exon/intron borders of DJ-1, since variations have been reported in these regions [22]. We detected a heterozygote Ala167Ala mutation in two out of 144 Swedish PD patients, but no Ala167Ala carriers were identified among the 213 neurologically healthy controls. This is the first report on DJ-1 mutations identified in a Swedish PD material. The Ala167Ala mutation was previously identified in a PD patient (homozygote carrier) from the United Kingdom and in a patient from a North American material with Caucasian/Hispanic origin (heterozygote carrier) [22,23]. One of the Swedish Ala167Ala carriers was diagnosed with Alzheimer’s disease as well (Table 1), which is in agreement with the reports that DJ-1 has been implicated in Alzheimer’s disease [8]. Mechanisms by which the synonymous Ala167Ala mutation may have consequences on the protein level and predispose to disease are not well understood. The DJ-1 protein has been suggested to act in multiple pathways and hypothesized that loss of the protective function of DJ-1 in dopaminergic neurons can lead to neurodegeneration [24]. Several possibilities have been suggested for synonymous mutations including alterations of mRNA stability, secondary structure, transcriptional activity, or changes in protein synthesis, folding, levels, turnover and/or function [25]. We found a small decrease in mRNA folding energy (1.3%) comparing the mutated Ala167Ala sequence with the wild type sequence, suggesting a possible effect of Ala167Ala on DJ-1 gene function [26]. However, no strong conclusions can be drawn from the artificial conditions used for modeling secondary structures in silico.
The frequency of DJ-1 mutations has been reported to vary among PD populations with different ethnic origin, Caucasian: 0.91%, Asian: 3.03%, Arab: 0.74% and Ashkenazi Jews: 1.96% and copy number variations in DJ-1 have so far only been reported in the Caucasian PD population (0.54%) [16]. We found the Ala167Ala mutation in two PD patients in our Swedish cohort, but no other genetic variant in any of the three investigated exons. The observed DJ-1 mutation frequency (1.4%) is in agreement with findings in other Caucasian PD populations.
CONCLUSION
This is the first report on a DJ-1 mutation identified in Swedish PD patients. Based on the results of the screening in our Swedish cohort as well as the results from other studies, it appears clear that mutations in DJ-1 are a rare cause of familial and early-onset PD world-wide.
ACKNOWLEDGEMENTS
We would like to thank Professor Laura Fratiglioni for providing us with control samples from the Swedish National Study on Aging and Care in Kungsholmen (SNAC-K) project and Doctor Fengqing Xiang for excellent technical assistance. The study was supported by the Swedish Research Council, the Swedish Brain Foundation, Karolinska Institutet Funds, the Swedish Parkinson Foundation, Swedish Brain Power and Åhlen´s Foundation.


