All published articles of this journal are available on ScienceDirect.
Further Electrochemical and Behavioural Evidence of a Direct Relationship Between Central 5-HT and Cytoskeleton in the Control of Mood
Abstract
Reduced activity of CNS serotonin is reported in unipolar depression and serotonin is the major target of recent antidepressant drugs. However, an acute depletion of serotonin in healthy individuals does not induce depressive symptoms suggesting that depression does not correlate with the serotonin system only. Neuronal plasticity (structural adaptation of neurons to functional requirements) includes synthesis of microtubular proteins such as tyrosinated isoform of α-tubulin and presence of serotonin as regulator of synaptogenesis. In depression neuronal plasticity is modified.
Here, in rats submitted to a behavioural test widely used to predict the efficacy of antidepressant drugs (forced swimming test: FST) a significant decrease of both cerebral tyrosinated α-tubulin expression and serotonin levels is monitored. Moreover, treatment with para-chlorophenylalanine (PCPA, compound that specifically depletes brain serotonin) but not alpha-methyl para tyrosine (α-MPT, compound that blocks synthesis of catechols: chemicals also implicated in depression) significantly reduced tyrosinated α-tubulin. Thus, a direct relationship between serotonin and tyrosinated α-tubulin appears to be present both in “physiological” and in “pathological” states. In addition, data obtained in animals submitted to FST and/or treated with the selective serotonin reuptake inhibitor (SSRI) fluoxetine further support the interrelationship between central serotonin and cytoskeleton. These data propose that direct relationship between serotonin and tyrosinated α-tubulin could be considered within the mechanism(s) involved in the pathogenesis of depression.
1. INTRODUCTION
The cytoskeleton is the structural component of neurones and three polymers, i.e. microtubules, intermediate filaments and microfilaments are their components [1].
Microtubules derive from the polymerisation of tubulin proteins (heterodimers consisting of α- and β- sub-units), they are abundant in neurones and exhibit high heterogeneity of components. Furthermore, microtubules are highly dynamic polymers as they exchange rapidly the polymerised tubulin dimers with soluble sub-unit pools. This feature is involved in fundamental cellular functions such as cell division, motility and transport, maintenance of cell shape and signal transduction [2].
The α-tubulin sub-unit of the microtubule is expressed in mammalian species in various isoforms related to different primary sequences and several post-translational modifications. One of these modifications is the cyclic tyrosination / detyrosination of the C-terminus, cycle that has been shown to occur in many cell types and to be related to the microtubule dynamic [3]. Stable microtubules are detyrosinated [4], whilst the highly dynamic microtubules show to be highly tyrosinated [3].
High levels of tyrosinated α-tubulin (Tyr-tub) are currently used as a marker of dynamic forms of microtubules [3, 5, 6]. Its expression is increased in physiological events such as the development of the central nervous system [7, 8] as well as pathological events such as experimental epileptogenesis, characterised by neuronal plasticity [9].
Neuronal plasticity is the capability of neurones to modify their structural organisation in order to adapt to new functional requirements. It plays a crucial role within brain development and in many physiological processes of mature brain such as learning and memory [10]. Neuronal plasticity requires the presence of serotonin (5-HT) as regulator of synaptogenesis [11] and cytoskeletal states necessary for synaptic remodelling i.e. dynamic form of microtubules [12]. Indeed, a more dynamic microtubule (major presence of soluble form versus insoluble form) is essential for rapid intracellular events resulting in elongation or shortening of cell processes, which are essential morphogenetic events during neurogenesis [1].
Recently, basic and clinical studies have provided direct evidence of neuronal plasticity failure in response to stress and depression that leads to neuronal atrophy, cell death and neurogenesis decrease in the hippocampus [13-16].
Several neurotransmitters have been implicated in the pathogenesis of depression. Reduced activity of monoamines such as 5-HT and noradrenaline (NA) systems has been reported in sub-groups of patients with major depression [17] and antidepressant drugs act mostly via increased amount of such neurotransmitters in the synaptic space.
To date the 5-HT system is one of the major targets of antidepressant drugs and Selective Serotonin Reuptake Inhibitors (SSRIs) are highly effective, producing milder side effects than tricyclic antidepressant drugs [17-21].
However, depletion of 5-HT or NA in healthy subjects does not induce clinically significant depressive symptomatology. Thus, abnormalities in other neurobiological systems may play a role in depressive symptomatology [17]. For instance, various studies have proposed that the increased central 5-HT levels following a long-term antidepressant treatment activate several post-receptor signalling pathway and in particular that of Protein Kinase A (PKA) in microtubules, resulting in the inhibition of microtubule assembly [12, 22-24].
The consequent major dynamic instability of the microtubules may favour the cytoskeletal changes necessary for long-term (plastic) modifications in neurotransmission [25]. Taken together with the neuronal plasticity failure observed in stress and depression, these findings support the cytoskeleton as one of the systems involved in depression.
In the present study, that follows preliminary work presented to the 15th ECNP Congress [26], experiments have been performed in order to monitor a putative interrelationship between cytoskeleton and cerebral monoamines. In particular, the influence of pharmacological depletion of catechols or serotonin (5-HT) upon Tyr-tub levels has been analysed. Naive rats were treated either with α-methyl-para-tyrosine (αMPT) that blocks synthesis of catechols [27, 28] or with para-chlorophenylalanine (PCPA) a tryptophan hydroxylase inhibitor that conspicuously decreases central 5-HT content without altering 5-HT terminal density [29, 30].
Finally the FST: an animal model widely used to predict the efficacy of antidepressant drugs as it is stated by the first author proposing such behavioural model [31] and then adopted by the researchers of the field, was applied either alone or in presence of treatment with fluoxetine. Therefore this model considered as “predictor” of the efficacy of antidepressant drugs such as the SSRIs was used in order to analyse the two systems studied i.e. 5-HT and cytoskeleton, within either a condition that could mimic a “depressive state” and following treatment with an “antidepressant drug“.
2. . METHODS
2.1. . Animals
Adult male Sprague Dawley rats (250-300g) were used. All housing and experimental procedures were carried out in accordance with the Italian law (Legislative Decree no.116, 27 January 1992), which acknowledges the European Directive 86/609/EEC, and were fully compliant with GlaxoSmithKline policy on the care and use of laboratory animal and codes of practice. Furthermore, all efforts were made to minimize the number of animals used and their suffering.
2.2. Treatments
Adult male Sprague Dawley rats (250-300g) were treated with:
- PCPA (500mg/kg i.p. n=4) or
- with αMPT (250mg/kg i.p. n=4) or
- vehicle (NaCl 0.9% 2ml/kg i.p., n=4, control group). 24 hours later rats were anesthetised with chloral hydrate (400mg/kg i.p.), then were sacrificed, the brains were removed and homogenised in lysis buffer.
Subsequently, concomitant electrochemical analysis of both DA and 5-HT levels (using differential pulse voltammetry, see below) and Tyr-tub expression (western blot) were performed in each brain homogenate. - In other animals, acute treatment with fluoxetine (SSRI 20mg/kg i.p., n=4) or vehicle (NaCl 0.9% 2ml/kg i.p., n=4, control group) was performed. Two hours later rats were anesthetised with chloral hydrate (400mg/kg i.p.), then were sacrificed, the brains were removed and homogenised in lysis buffer.
Subsequent analysis of both 5-HT levels (voltammetry, see below) and Tyr-tub expression (western blot) were performed.
2.3. Western Blot Procedure
Tyr-Tub expression was evaluated in brain homogenate by densitometric quantification of related band previously obtained via western blot.
In particular, brains were homogenised in lysis buffer [5mM Tris-HCl, 2mM EGTA, 0.1mM phenylmethylsulfonyl fluoride (PMSF), 0.1mM pepstatin A, 1 mM leupeptin, 1mM aprotinin, pH 8.0]. Protein concentrations were determined via colorimetric assay (Bradford protein assay; Bio-Rad Hercules, CA, USA), setting the spectrophotometer to 595 nm. Samples (10µg of total proteins) were diluted in sample buffer [62.5mM Tris-HCl pH 6.8, 20% glycerol, 2% sodium dodecyl sulfate (SDS), 5% β-mercaptoethanol, 0.5% bromophenol blue (BPB)]. Protein samples were heated for 3 min at 99°C and proteins were separated on SDS - 12% polyacrylamide gel electrophoresis, at 40 mA for 1hr and then transferred onto nitrocellulose membranes using a dry transfer unit at 50mA overnight. A molecular size marker (200-6.5kD range; Bio-Rad) and pure tubulin (ICN Costa Mesa, CA, USA) were used in order to exactly locate tubulin (55kD) on the basis of its position on the gel. The membranes were blocked for 2 hours with 5% skimmed powder milk in Tris-buffered saline (TBS) and incubated 1hr at 4°C with monoclonal antibodies clone TUB-1A2 (Sigma) specific to Tyr-Tub at 1:1000 dilution in 0.1% skimmed powder milk in TBS. The membranes were then incubated with a 1:5000 IgG anti-mouse alkaline phosphatase antibody (Promega Madison, WI, USA) for 30 min and the reaction developed using a stabilized substrate for alkaline phosphatase (Western Blue; Promega). The immunoreactivity of the Tyr-Tub bands obtained was quantified by densitometry (Molecular Analyst Software; Bio-Rad).
2.4. . Voltammetry and Carbon Fibre Micro Electrodes (Micro-Biosensors)
Within the different voltammetric methodologies available, Differential Pulse Voltammetry (DPV, that is a linear voltammetric technique with superimposed constant pulses (△V: 50mV; t: 50ms) has been selected since this technique exhibits both a high selectivity and sensitivity (for a review see Stamford et al. [32]). DPV was applied by means of the polarograph µAUTOLAB (EcoChemie, The Netherlands) linked to IBM computer with voltammetric analysis program (General Purpose Electrochemical System Software package (GPES). The three-electrode potentiostat system needed to apply voltammetry was prepared as described previously [32, 33]. Briefly, the reference electrode was silver/silver chloride (Ag/AgCl) and the auxiliary (counter) electrode was a silver wire, both approximately 100µm diameter. The working electrode was the 30µm diameter mCFE prepared and treated as previously described in order to increase the sensitivity, selectivity and reliability of the micro-sensor (34). Briefly, a 70 Hz triangular wave form was applied in three stages, 0 to +3 Volts for 8 sec.; 0 to +2.6 V and 0 to +1.5 V for 10s each. Then, two successive continuous potentials were applied to the mCFE: +1.45V and -0.9V, 5 s each. This electrochemical treatment was carried out with the auxiliary, reference and working electrodes immersed in 0.1M phosphate-buffered saline (PBS) at pH 7.4. It affects the active part of the working electrode i.e. the tip of the protruding carbon fibre from the end of the glass pipette (0.5-1.0 mm length; 30µm diameter). Then the tip was coated with Nafion as described in details by Crespi et al. [34]. Briefly: the tip of the mCFE was immersed four times (approximately 2 sec each time) into a Nafion drop (10µl of 5% solution, Solution Technology, Mendehall, PA) placed into a loop (about 3 mm diameter) formed at one end of a platinum wire of 100µm in diameter. The other end of the wire was connected to the reference and auxiliary outputs of the polarograph and a d.c. potential of + 3.5 V was applied. The Nafion-coated mCFE was then dried at 60°C for 20 sec and used immediately for the experiments. This electrochemical treatment was shown to increase sensitivity, selectivity and reliability of the micro-sensor to quantification of dopamine and noradrenaline [catecholamines, both having similar oxidation potential value] and serotonin [34-36].
The following measuring DPV parameters were chosen: initial potential (Ei) -100 mV, final potential (Ef) +350mV to monitor catecholamines and 5-HT in the same scan voltammogram. Scan step was 10mV•s-1 scan duration, 30s, filter 0.1 Hz. Other polarographic parameters employed were chosen according to Crespi et al. [34].
2.5. . Forced Swimming Test
Other groups of naive rats were submitted to FST as described by Porsolt et al. [31], except that the water was deeper [37]. Briefly, each animal was placed in a cylinder (40cm height; 18cm diameter) containing 30cm water maintained at 26-28°C. The modification of FST (30 cm water deep) avoids false immobility due to the capability of adult rats to rest on the tail when touching the bottom of the cylinder in a lower volume i.e. 15 cm as described by Porsolt et al. [31].
In a recent paper, the influence of “cold” water upon rat behaviour and in particular immobility within FST was discussed [38]. In our hand rats submitted to FST with water at the temperature of 26-28°C were showing significant different behaviour immobility in function of different treatments (see Results and Discussion).
After a pre-test session (day 1) of 15 min each rat was dried (under warm air current) and twenty-four hours later exposed again to FST during 5 min.
Both saline and fluoxetine sub-chronic treatment was performed 23h, 5h and 1h prior to the FST as previously described [37, 39]. The behaviour was monitored during the first 5 min of the pre-test and the following day for all the five 5 min of the FST.
A time-sampling method was used as described previously [37] in order to score several behaviours during a single viewing. This method has been selected as it has shown to be reliable and valid for detecting the effect of different antidepressant drugs. In particular, immobility and swimming were monitored in 5-sec period. Briefly, immobility was scored when the animal was making the minimum movements necessary to keep its head above water and stay afloat. Swimming was scored when the animal actively swam around the tank, making movements greater than that necessary to stay afloat.
Three groups of animals (n=4 each group) were submitted to FST: untreated rats (naive, n=4) or rats pre-treated with vehicle (NaCl 0.9%) or fluoxetine as described above.
Immediately after FST, each rat was sacrificed, the brain was removed and homogenised in lysis buffer so that ex vivo parallel experiments could be performed to monitor 5-HT levels and Tyr-Tub expression. Briefly, 5-HT levels were selectively measured within 200µl sample of brain homogenate using DPV associated with carbon fibre microelectrodes (mFE) coated with Nafion [34]. In the same brain homogenate assessed with DPV-mCFE the Tyr-Tub (55 kDa) western blot immunoreactivity was performed and quantified by densitometric analysis.
2.6. . Drugs
PCPA, αMPT, chloral hydrate, (Sigma St. Louis, MO, USA), fluoxetine (Tocris); Nafion (Aldrich).
2.7. . Statistical Analysis
The data obtained from all the experiments were analyzed using analysis of variance (ANOVA) and the post-hoc test were conducted using Dunnett’s test.
3. RESULTS
3.1. . Pharmacological Treatments
- PCPA treatment resulted in a significant decrease of Tyr-Tub expression in the whole brain (approximately 75% of vehicle treated rats (NaCl 0.9% 600 µl i.p., control rats) (Fig. 1 TOP).
- In contrast, no significant changes were monitored in αMPT treated rats (Fig. 1 BOTTOM).
Concomitant DPV measurements in the same homogenate resulted in a large decrease to approximately 45% or 12% of control values of both the catecholaminergic (Peak 1) and the 5-HT (Peak 2) related signals, respectively (Fig. 1 right, see also Fig. 2 for technicality). - Acute fluoxetine (20 mg/kg i.p.) resulted in a significant increase of both 5-HT levels and Tyr-tub expression in whole brain up to approximately 217±40% and 203±24%, respectively, of control rats treated with NaCl 0.9% (vehicle) 600 µl i.p. (Fig. 3).
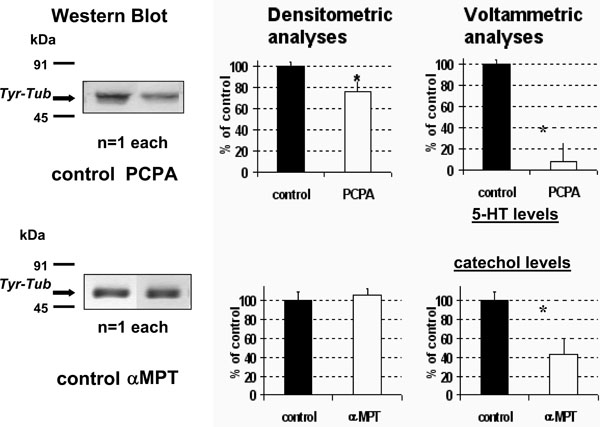
Tyr-Tub (55kDa) immunoreactivity in brain homogenate: western blot bands (left) quantified by densitometric analysis and concomitant DPV measurements (right) after acute treatment with PCPA 500mg/kg i.p. (TOP) or with αMPT 250mg/kg (BOTTOM). Results are expressed as percent of control (vehicle treated rats); n=4 each histogram, mean ± S.D. *p<0.05, Dunnett’s test. Note the significant decrease, (F1,12= 7,5264 p<0.05) of Tyr-Tub following PCPA versus αMPT treatment.
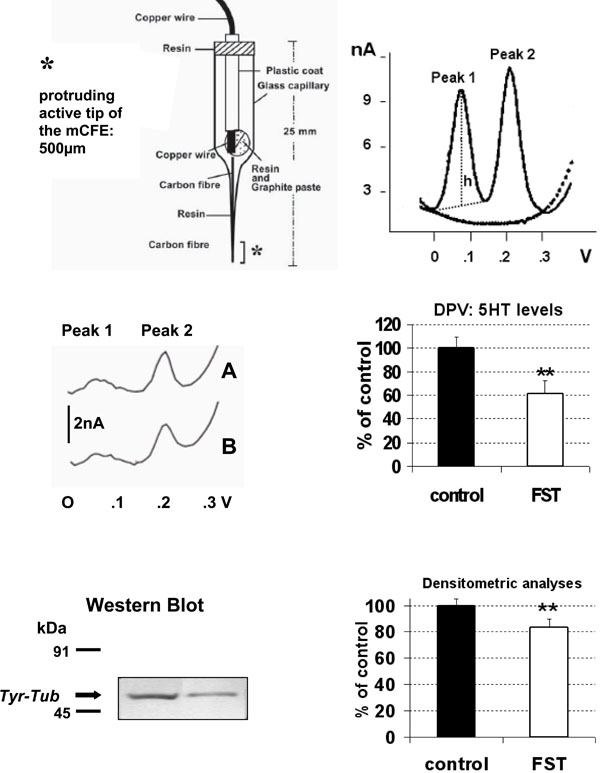
TOP LEFT: schematic representation of the carbon fibre microelectrode used in the present experiments.
TOP RIGHT: differential pulse voltammogram obtained in vitro with Nafion mCFE: dotted line shows signals obtained in phosphate buffer solution (PBS, pH 7.4), solid line shows Peak 1 at approximately 69mV and Peak 2 at approximately 230mV corresponding to the oxidation of catecholamines [dopamine or noradrenaline] and 5-HT, respectively (50nM in PBS).
h: height of the peak, measured in nanoAmperes (nA: Intensity of current). This perpendicular line also determined the exact potential value expressed in Volts (V) of each signal on the abscissa.
MIDDLE: differential pulse voltammogram obtained in vitro with Nafion mCFE within 200µl sample of the brain homogenate of a naïve (A) rat or a rat submitted to FST (B) (LEFT) and in six rats each group (RIGHT).
BOTTOM: In the same 200µl sample brain homogenate the Tyr-Tub (55 kDa) western blot immunoreactivity was performed (left) and quantified by densitometric analysis (right).
Results are expressed as percent of control (naive rats); n=6 each histogram, mean ± S.D. **p<0.01, Dunnett’s test.

Effect of acute treatment with fluoxetine alone (20 mg/kg i.p. n=4) or vehicle alone (NaCl 0.9% 2ml/kg i.p., n=4, control group) upon voltammetric 5-HT levels or Tyr-tub expression in rat whole brain. Both parameters were significantly increased: F1,8= 8.202 p<0.05) and F1,7= 7.727 p<0.05, respectively.
Data are expressed as % of control (vehicle), mean± S.D. *p<0.05, Dunnett’s test.
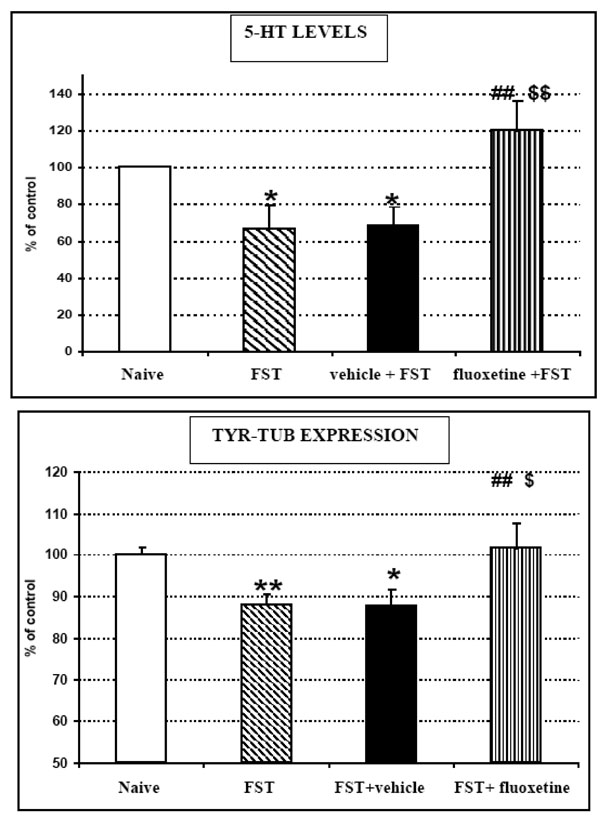
TOP: 5-HT levels monitored by means of ex vivo DPV and BOTTOM: Tyr-Tub (55 kDa) western blot immunoreactivity recorded in brain homogenates of naive (unstressed) rats or rats submitted to FST, FST + vehicle treatment or FST + fluoxetine treatment, respectively.
Results are expressed as percent of control (naive rats); n=5 each histogram,
mean ± S.D.
Stats for 5-HT data:
ANOVA treatment effect: F3,20= 5.52504 p=0.0078
*p<0.05 Unstressed versus FST or FST+ vehicle (Fisher's LSD Test)
##p<0.01 FST versus FST+fluoxetine (Fisher's LSD Test)
$$p<0.05 FST+ vehicle versus FST+fluoxetine (Fisher's LSD Test)
Stats for Tyr-Tub data:
ANOVA treatment effect F3,54= 4.87733 p=0.0047
*p<0.05 **p<0.01 Unstressed versus FST or FST+vehicle (Fisher's LSD Test)
##p<0.01 FST versus FST+fluoxetine (Fisher's LSD Test)
$p<0.05 FST+vehicle versus FST+fluoxetine (Fisher's LSD Test)
*p<0.05 Unstressed vs FST or FST+saline (Fisher's LSD Test)
##p<0.01 FST vs FST+fluoxetine (Fisher's LSD Test)
$$p<0.05 FST+saline vs FST+fluoxetine (Fisher's LSD Test)
3.2. . Forced Swimming Test
- FST significantly increased immobility counts from 19.8±1.5 (untreated rats) or from 16.8±1.0 (vehicle treated rats) recorded in the pre-test day, up to 31.4±3.9 or to 31.0±2.1 gathered in day 1, respectively (mean ±SEM) (see Table 1).
In fluoxetine treated rats submitted to FST, immobility latency appeared to be increased (approximately 45s) while immobility counts were significantly decreased (22.8±2.7, mean ±SEM) when compared to untreated rats (31.4±3.9) or vehicle treated rats (31.0±2.1). Furthermore, fluoxetine treated rats showed higher swimming behaviour and active behaviour counts when compared to untreated or vehicle treated rats. In contrast, climbing behaviour counts were similar in all animals tested (Table 1). - Immediately after FST, rats were sacrificed. The brains were immediately removed and homogenised. Successive concomitant electrochemical [voltammetric] and western blot analyses were performed in the whole brain homogenate of rats submitted to FST and showed that:
- In vehicle treated rats 5-HT levels and Tyr-tub expression were both significantly decreased by FST to approximately 69±11% and 87±4% of corresponding levels monitored in naive control rats (Fig. 4). These data are in accord with the results gathered from the previous groups of animals submitted to FST and then tested for Tyr-tub expression and 5-HT levels as described in Fig. (2) middle and bottom.
- In fluoxetine treated rats submitted to FST, 5-HT levels and Tyr-tub expression reached 120±16% and 103±5% of corresponding levels monitored in naive control rats, respectively. On the other hand, when compared to data gathered from vehicle treated rats submitted to FST and considered 100%, these differences were approximately 150% and 116%, respectively (Fig. 4).
- in naive rats submitted to FST only, changes in 5-HT levels and Tyr-tub expression were very close to those monitored in vehicle + FST treated rats (Fig. 4).
| BEHAVIOUR | Untreated Rats | ANOVA | |
|---|---|---|---|
| pre-test | FST | ||
| immobility latency (sec) | 114 ± 18 | 67 ± 11 | F1,9=5.07 p=0.0543 |
| immobility (counts) | 19.8 ± 1.5 | 31.4 ± 3.9* | F1,9= 7.47 p=0.0257 |
| swimming (counts) | 21.8 ± 2.5 | 11.4 ± 3.6* | F1,9=5.38 p=0.0489 |
| climbing (counts) | 16.6 ± 3.3 | 17.2 ± 3.7 | F1,9=0.01 p=0.9082 |
| diving (counts) | 1.8 ± 0.8 | 0 | F1,9=5.06 p=0.0546 |
| active (counts) | 40.2 ± 1.5 | 28.6 ± 3.9* | F1,9=7.47 p=0.0257 |
| Vehicle Rats | ANOVA | ||
| pre-test | FST | ||
| immobility latency (sec) | 110 ± 17 | 65 ± 8* | F1,9=5.95 p=0.0405 |
| immobility (counts) | 16.8 ± 1 | 31 ± 2.1** | F1,9= 34.52 p=0.0004 |
| swimming (counts) | 19.4 ± 3.7 | 14.8 ± 2 | F1,9=1.13 p=0.3173 |
| climbing (counts) | 21 ± 2.9 | 14.2 ± 1.6 | F1,9=4.15 p=0.076 |
| diving (counts) | 2 ± 1.2 | 0 | F1,9=2.5 p=0.1525 |
| active (counts) | 42.4 ± 1.6 | 29 ± 2.1** | F1,9=24.39 p=0.0011 |
| FLUOXETINE Rats | |||
| pre-test | FST | ANOVA | |
| immobility latency (sec) | 124 ± 19 | 112 ± 19 | F1,9=0,60 p=0.4579 |
| immobility (counts) | 17.6 ± 1.9 | 22.8 ± 2.7 | F1,9= 2.90 p=0.1266 |
| swimming (counts) | 19.2 ± 5 | 20.6 ± 2.7 | F1,9=0.06 p=0.7987 |
| climbing (counts) | 22.6 ± 3.2 | 15 ± 3.4 | F1,9=2.45 p=0.156 |
| diving (counts) | 1.8 ± 1.2 | 0 | F1,9=2.25 p=0.172 |
| active (counts) | 43.6 ± 3.3 | 35.6 ± 2.8 | F1,9=3.80 p=0.0868 |
| POST-HOC= *p<0.05 **p<0.01 pre-test vs FST (Dunnett's Test) | |||
DISCUSSION
Earlier in vitro studies have indicated that long-term antidepressant SSRI treatment inhibits microtubule assembly via PKA pathway activation [12]. This suggests that increased 5-HT levels (due to chronic SSRI treatment) precede the inhibition of microtubule assembly and therefore the increase of its dynamic instability.
Our curiosity was then to analyse the possibility that these two systems could be interrelated and that changes in central serotonin could drive cytoskeleton modifications. This hypothesis, firstly indicated in a presentation to the 15th ECNP Congress Barcelona - Spain, 5 - 9 October, 2002 [26] has been further tested via various analytical approaches:
1. Treatment with PCPA or α-MPT
These are compounds that selectively deplete central 5-HT or catechols, respectively [40, 41]. The present data indicate that only depletion of central 5-HT by PCPA resulted in significant changes in Tyr-tub levels. PCPA could have an independent action upon the cytoskeleton. On the other hand these data may support a putative interaction between central 5-HT system and cytoskeleton dynamics while indicating absence of such interaction for what concerns the catecholaminergic system.
2. Acute Treatment with Fluoxetine (SSRI)
This was performed in order to influence the central serotonergic system and to verify putative subsequences on both 5-HT levels and Tyr-tub expression. This resulted in a significant increase of both 5-HT levels and Tyr-tub expression in whole brain up to approximately 217% and 203% of saline treated rats, respectively.
Up to date the 5-HT system is still one the major target of antidepressant SSRIs [17-21] thus the variation of 5-HT levels was to be expected.
Additionally, it is known that fluoxetine is a lipophilic chemical that can be located in subcellular fragments prepared from brain tissue of fluoxetine-treated rats [42]. Therefore, fluoxetine may enter the cell and interact with monoamine oxidase (MAO), enzyme present in both neurons and glia and that metabolises the classical monoamine neurotransmitters [43]. Consequently, the therapeutic actions of fluoxetine may [also] involve an inhibition of such enzyme and in particular the form MAO-B, which would result in a greater amount of 5-HT available for release as indeed already reported [44]. Furthermore, in vitro and in vivo study have shown that doses of 20 mg/kg produced a more-than-20% decrease in MAO A activity and a 10–15% suppression of MAO B activity in the rat brain [45, 46].
Clinical studies have also shown that fluoxetine and other SSRI antidepressants, used at low dosage are beneficial in psychiatric disorders because they increase the availability or potency of neuroactive GABAergic steroids [for reviews see 47-49]. It appears that their ability to increase brain steroid biosynthesis is occurring at doses [i.e. 5mg/kg in rats] devoid of significant action on brain 5-HT reuptake mechanisms [50, 51].
Therefore they are proposed as new class of pharmacological tools for the management of anxiety, related mood disorders, dysphoria, and impulsive aggression so called “selective brain steroidogenic stimulants” (SBSSs). The molecular mechanisms subserving the fluoxetine induced facilitation on neurosteroidogenesis remain to be investigated and defined in terms of structural chemical related specificity in activating brain steroidogenesis, which is a novel and intriguing branch of psychopharmacology.
In our work, the dose of fluoxetine used is superior to the 5mg/kg and already proven to act on the changing of monoamine concentrations at the synapses via the selective inhibition of 5-HT reuptake. Nevertheless, these two components of fluoxetine central mechanism of action have to be considered when regarding its influence on cytoskeletal dynamics. It is definitely known that some neurosteroids have a direct action on cytoskeletal protein such as as the microtubule associated protein 2 (MAP2) that has an important role in neuronal morphogenesis stimulating for instance the extension of neurites [52].
Indeed, results suggest that MAP2 expression may be necessary for both neurite extension and cessation of cell division as the specific suppression of MAP2 synthesis prevents the neuronal differentiation of embryonic carcinoma cells exposed to retinoic acid, as shown by the absence of neurites [53].
On the other hand, the proposed modification of Tyr-tub is an original observation. It could be due to an SSRI direct action on the cytoskeleton and/or it could be successive to changes on central serotonin activity, opening to the hypothesis of an interrelationship between these two systems.
3. . FST
Such postulation was also challenged in animals submitted to FST following vehicle or fluoxetine treatment as described by Porsolt et al. [31]. FST is an animal model of depression widely used to predict the efficacy of antidepressant drugs [31]. Successively ex vivo analysis of brain 5-HT levels and Tyr-tub expression were performed. These two latter measurements were also carried out in control naive rats.
The pre-test session of the FST (15 min swimming, day 1) induces a state of behavioural despair that is thought to increase the duration of immobility during the 5 min swimming test session of day 2 (FST) [39]. Accordingly, here the rats submitted to FST did show a significant (p<0.05 Dunnett’s test) increase of immobility up to approximately 158% of the values gathered in day 1 (pre-test day). In addition, during the FST, the fluoxetine treated rats displayed significantly lower immobility and higher swimming counts when compared to saline treated rats. Climbing counts were similar in both type of treatment (Table 1). In the successive parallel ex vivo electrochemical and western blot studies in whole brain it was observed that:
- In saline treated rats 5-HT levels and Tyr-tub expression were both decreased to approximately 69% and 88% of corresponding levels monitored in naive control rats, respectively.
- In fluoxetine treated rats 5-HT levels and Tyr-tub expression were both increased up to approximately 120% and 104% of corresponding levels monitored in naive control rats, respectively. On the other hand, when compared to data gathered from vehicle treated rats, these differences were approximately 150% and 120%, respectively.
In addition, naive rats were submitted to FST in order to analyse the putative influence of systemic injection of vehicle (NaCl 0.9%, 600µl i.p.) upon the parameter measured. The data gathered within the naive rats were very similar to those observed in vehicle treated rats (see Table 2). Thus, injection of vehicle did not alter significantly the effect of FST upon 5-HT levels and Tyr-tub expression. Therefore, this data indicate that the reduction of 5HT levels and Tyr-tub expression following FST is not altered significantly by the manipulation of the animal when submitted to i.p. treatment, thus supporting both these reductions as directly related to the FST and independent from the putative stressful manipulation of the animal due to the systemic injection.
- Naïve control rats (n=5) that were not submitted to FST neither injected.
- Untreated rats (n=5) that were submitted to FST.
- Vehicle + FST rats (n=5) that were submitted to FST after sub-chronic treatment with saline (600ml i.p.) as described in Methods.
Data are expressed as mean±sem;
* p<0.05: naïve unstressed versus FST or vehicle + FST ((Fisher's LSD Test).
These original parallel electrochemical and western blot results further support such combined methodology as useful concomitant analytical approach indicating that in rats submitted to FST a reduced neuronal plasticity can be detected together with coupled decreased activity of central serotonergic system. They are in agreement for instance and therefore corroborate with separate experiments showing i) the neuronal plasticity failure [13-15] and ii) the reduced central 5-HT levels in condition of depression [17].
All these data support a strict relationship between 5-HT and cytoskeleton as the two systems are modified in a parallel way in all the different types of treatments. Again, the pharmacological or behavioural induced changes of serotonergic levels were expected as already reported in the literature, while the original observation that FST modifies Tyr-tub expression may lead to the hypothesis of a primary influence of the central 5-HT system upon the cytoskeleton condition. It has been shown that different acute stressful conditions [i.e. 5-min forced swim in cold water] result in hyperphosphorylation of microtubule-associated proteins such as TAU [54] and that neuronal TAU hyperphosphorylation decreases microtubule dynamics resulting in neuronal plasticity impairment [55].
On the other hand, fluoxetine was reported to have the ability to reverse stress-induced changes [56, 57] and that fluoxetine increases the phosphorylation of MAP-2 [58] that is amongst neuronal microtubule-associated proteins (MAPs) an abundant group of cytoskeletal components. This activity promotes microtubule dynamics [59] resulting in more Tyr-Tub expression [5, 26]. In the literature this type of studies is generally performed in the hippocampus, the evidence in our work that such effect of fluoxetine is detected in the whole brain may indicate a general activity over the CNS of such compound.
In conclusion, the present work supports the preliminary hypothesis about a direct serotonin and neuronal plasticity relationship [26] as it proposes that changes in central 5-HT levels obtained either within a behavioural test resulting in stress and/or depression states (FST) or via selective pharmacological treatments (i.e. PCPA, fluoxetine treatment) have direct influence upon the microtubular network resulting in modified activity of the neuronal plasticity.
In particular, the original hypothesis that the decreased 5-HT levels observed in depression [17] may be selectively correlated to a reduced dynamic instability of the microtubular network could be formulated.
Finally, the direct relationship observed between 5-HT and the microtubular network can be proposed as a mechanism involved in the control of mood which may be an innovative target for alternative therapeutic approaches to treat depression.
ACKNOWLEDGEMENTS
For technical support to Dr. E. Vecchiato and Dr. C. Lazzarini.


