Serum Ferritin and Metal Levels as Risk Factors for Amyotrophic Lateral Sclerosis
Abstract
Metal toxicity has been identified as a possible risk factor for amyotrophic lateral sclerosis (ALS) and other neurodegenerative disorders. We conducted a retrospective chart review of urinary, hair and blood metal levels and serum ferritin in 321 people with ALS seen over a ten-year period at the Massachusetts General Hospital (MGH). We found that hair lead levels and serum ferritin levels were elevated in ALS patients compared to published normal values. Metal levels of arsenic, lead, mercury, cadmium, thallium, cobalt and aluminum in 24-hour urine specimens and lead, mercury and arsenic in serum were within the normal range. We conclude that twenty-four hour urine or blood testing for metals is not warranted as part of the evaluation of ALS. Elevated levels of serum ferritin in ALS population could reflect an underlying perturbation in iron metabolism.
INTRODUCTION
Amyotrophic lateral sclerosis (ALS) is a fatal neurodegenerative disorder that affects predominantly motor neurons. The cause of the sporadic form of ALS is unknown [1, 2]; metal toxicity has been proposed as a possible risk factor [3, 4]. Several approaches have been taken to investigate metal toxicity in ALS, including: measuring metal levels in body tissues and fluids; comparing case-control cohorts to establish the risk of contracting ALS from metal exposure; and evaluating case reports of clinical syndromes resembling ALS that developed following metal exposure. The possibility that people with ALS might have a genetic susceptibility to the effects of metals has also been studied [5]. Serum ferritin levels reflect both inflammation and iron imbalance in defined pathophysiological conditions such as hemochromatosis (iron) and the anemia of chronic disease (inflammation) [6-8]. We conducted a retrospective analysis of measurements of metals in urine, blood and hair samples and tested serum ferritin levels in ALS compared to age matched subjects.
METHODS
A retrospective chart review of 321 people with ALS seen at the Neuromuscular Clinic at the Massachusetts General Hospital (MGH) between January 1994 and December 2003 was performed. Approval for the chart review was obtained from the MGH Institutional Review Board. Participants were selected if they were evaluated for at least one laboratory test related to metal level in serum, hair or urine. All participants were identified using the computerized MGH Research Patient Data Registry (RPDR) and each participant’s diagnosis was confirmed according to the El-Escorial criteria [9]. Participant’s gender, date of birth, site and date of symptom onset and date of diagnosis were recorded. The participants were selected randomly based on availability of outpatient medical records. All patients whose complete medical record file was maintained at the Outpatient Clinic were included in the chart review. The laboratory samples collected from the patients were analyzed at the Nichols Institute in Chantilly, Virginia. Normal reference ranges for metals were available and had been obtained from published data and private internal testing of healthy volunteers at the Nichols Institute [10-12].
After the initial chart review, additional stored serum samples from 30 participants with ALS and 30 healthy control volunteers who participated in an ALS observational study from 1998 to 2002 [13] were analyzed for ferritin levels. These samples were sent to the Pathology Laboratory at MGH and analyzed using an electrochemiluminescent immunoassay, ELECSYS 2010 (Roche Diagnostics, Indianapolis, IN).
Statistical analyses were performed using SAS version 8.0 (Cary, NC), and a univariate analysis were used to analyze the data. Student’s T-test was used to compare ferritin levels between ALS patients and controls.
RESULTS
The majority of participants were male (59%) and the mean age at symptom onset was 56.3 years (± 13.0 SD). The number of participants who underwent each laboratory test and the normal reference ranges are provided in Table 1. The mean 24-hour urine levels of arsenic, lead, mercury, cadmium, thallium, cobalt and aluminum were within the normal range (Table 1). Four participants had lead levels measured from hair. The average hair lead level was elevated (1.9 ug/gm ± 2.6 SD; normal range = 0 - 0.99 ug/gm). Serum ferritin levels were measured in 12 participants (six male and six females). The mean serum ferritin level was elevated in the participants with ALS. In males with ALS, the average ferritin level was 668 ng/ml (± 785.1 SD) compared to a normal range of 22 - 322 ng/ml. In female ALS patients, the average ferritin level was 237.2 ng/ml (± 180.3 SD; normal range 10 - 291 ng/ml).
Metal Levels in Blood, Hair and 24-Hour Urine Samples
| Laboratory Investigation | Number of ALS Subjects | Mean Level--ALS Subjects (units) | Standard Deviation (SD) | Normal Range |
|---|---|---|---|---|
| Urine | ||||
| Arsenic | 110 | 52.3 (mcg/L) | 66.1 | 0 – 80 mcg/L |
| Lead | 110 | 13.7 (mcg/L) | 10.9 | 0 – 50 mcg/L |
| Mercury | 112 | 8.9 (mcg/L) | 2.7 | 0 – 20 mcg/L |
| Cadmium | 98 | 0.9 (mcg/L) | 2.9 | 0 – 3 mcg/L |
| Thallium | 79 | 0.9 (mcg/L) | 1.8 | 0 – 2 mcg/L |
| Cobalt | 70 | 0.5 (mcg/L) | 0.1 | 0 – 2 mcg/L |
| Aluminum | 8 | 12.9 (mcg/24 hr) | 8.9 | 7 – 40 mcg/24 hr |
| Serum | ||||
| Lead | 86 | 4.2 (mcg/dL) | 2.9 | 0 – 25 mcg/dL |
| Arsenic | 6 | 10 (mcg/L) | 0 | 0 – 60 mcg/L |
| Mercury | 6 | 15.2 (ug/L) | 6.4 | 0 – 50 ug/L |
| Hair | ||||
| Lead | 4 | 1.9 (ug/GM) | 2.6 | 0 – 0.99 ug/GM |
| Arsenic | 5 | 0.2 (ug/GM) | 0.4 | 0 – 0.9 ug/GM |
| Mercury | 5 | 1.7 (ug/GM) | 1.1 | 0 – 24.9 ug/GM |
| Serum Ferritin | ||||
| Male | 6 | 668.0 (ng/mL) | 785.1 | 22-322 ng/mL |
| Female | 6 | 237.2 (ng/mL) | 180.3 | 10-291 ng/mL |
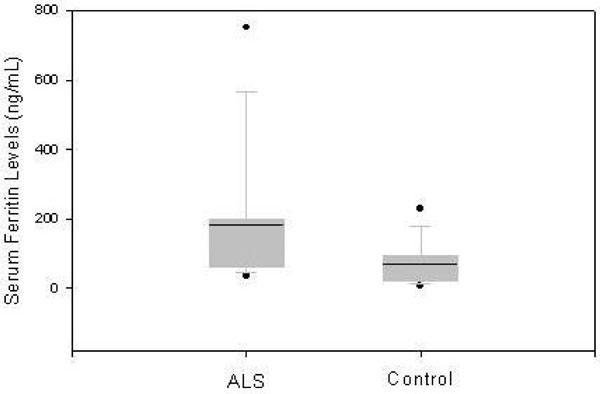
Based on these results we conducted additional analysis of serum ferritin levels in 30 individuals with ALS and 30 healthy controls. Compared with the healthy controls, the mean serum ferritin level in the ALS population was higher (ANOVA: males p=0.037, females p=0.032). The mean serum ferritin level in the 17 males with ALS was 269.9 ng/ml (± 126.4 SD), compared to 164.1 ng/ml (± 142.2 SD) in 14 healthy controls. The mean serum ferritin value in 13 females with ALS was 183.5 ng/ml (± 186.9 SD) compared to 71.3 ng/ml (± 60.4 SD) in 16 female control subjects (Figs. 1 and 2).

The mean serum ferritin level in 17 male participants with ALS (269.9 ng/ml ± 126.4 SD) and 14 healthy controls (164.1 ng/ml ± 142.2 SD) is depicted in boxplot. The horizontal line reflects the median and the box provides the interquartile range from the 25th to 75th percentile. Dots represent the observations outside of 10th and 90th percentiles.
The mean serum ferritin level in 13 female participants with ALS (183.5 ng/ml ± 186.93 SD) and 16 female controls (71.3 ng/ml ± 60.37 SD) is depicted in boxplot. The horizontal line reflects the median and the box provides the interquartile range from the 25th to 75th percentile. Dots represent the observations outside of 10th and 90th percentiles.
DISCUSSION
In a chart review of patients with ALS seen at MGH over a 10-year period we did not find evidence of increased metal levels in urine or blood compared to healthy controls. The hair lead levels were elevated in a limited number of participants who had this test performed. We also found significantly increased serum ferritin levels in both males and females with ALS. We confirmed this finding by measuring ferritin assays in a small set of stored serum samples.
Metal concentrations in blood and urine reflect recent exposures [14]; therefore measurements of metal levels in these tissues may not be useful except in clinical situations where a differential diagnosis between acute metal poisoning and a motor neuron disease is needed. The half-life of most metals in blood is usually measured in weeks [15]. By contrast, analysis of the metal content of hair is useful in assessing variations in long-term exposure to metals [16], especially vanadium [17], arsenic [18] and mercury [19]. However, several factors influence metal levels found in hair [20], and there is strong evidence of disparities between laboratories in hair metal measurements that cannot be attributed to the quality of laboratory methods [21, 22]. A recent study of hair metal levels in an ALS population found lower vanadium and manganese levels [17] but concluded that hair samples do not reflect exposures that may have occurred prior to the period in which the hair was growing. Chronic metal exposure can also be measured in bone. Since metals like lead and aluminum accumulate in the bones over the course of a lifetime, bone may be an endogenous source of these metals in the body [23]. The half-life of lead in the patella is three to five months, but in the tibia it is 15 to 25 years [15, 24]. Direct measurement of lead levels in bone provides data on cumulative past exposure that can be used in epidemiological analysis and clinical assessment [25].
The finding that hair lead levels were elevated in our cohort supports the earlier published data that demonstrated chronic elevation of bone lead in patients with ALS [24]. It has been shown that metals like zinc and cadmium regulate translation of ferritin by modulating binding of Iron-regulatory protein 1 (IRP1) to the Iron-regulatory element (IRE) in the 5’UTR of the ferritin mRNA [26]. It is possible that lead acts in a similar pathway to impact the synthesis of ferritin.
Elevated serum ferritin is an indicator of hemachromatosis and inflammatory stress [27]. The increased serum ferritin levels, as seen during inflammation [28], are induced by stress-induced factors such as interleukin-1 (IL-1) [29, 30]. Ferritin binds to iron and is involved in detoxifying iron and other trivalent trace metals in the brain [31]. In a transgenic mutation superoxide dismutase (SOD1) mouse model of motor neuron disease, neural ferritin expression was increased as a response to increased oxidative stress [32]. Defects in ferritin metabolism are possibly involved in etiology of other neurodegenerative deficits, including Friedrich ataxia [33], Parkinson’s disease [34], and Alzheimer disease [35]. Despite several reports of altered iron metabolism [36] and increased total brain ferritin in individuals with Alzheimer disease [37], their serum ferritin levels remain within the normal range [38].
Another crucial question concerns the potential role in ALS of polymorphisms in the hemachromatosis gene (HFE), located on the short arm of chromosome 6 at location 6p21.3 [39]. Two mutations in the HFE gene, H63D and C282Y, have been reported to increase susceptibility to ALS [40-42]. The HFE protein affects iron metabolism by binding to the transferrin receptor and reducing its affinity for iron-binding transferrin. However, the HFE allele can independently increase neuronal oxidative stress levels without necessarily affecting iron load [39]. The altered activity of SOD1 (either wild-type or the mutant SOD1G93A) modulates the levels of ferritin as well as transferrin receptor [43]. In light of these findings it will be important to further study the role of ferritin in in vitro and in vivo models of SOD1 toxicity.
The finding of elevated serum ferritin in a large sample of ALS population may simply be an acute phase reactant, rather than a specific maker of iron metabolism dysregulation. The role of ferritin in ALS needs to be studied in a longitudinal cohort and its relationship with survival and measures of disease progression should be explored. We conclude that further study of the role of ferritin and other proteins involved in iron metabolism is warranted in the ALS population, particularly in relation to genetic polymorphisms that affect cellular oxidative stress levels.
ACKNOWLEDGEMENTS
MEC receives support from the Digiovanni Fund. RHB receives support from the Angel Fund, ALS Therapy Alliance, Project ALS, the Al-Athel ALS Research Foundation, the Pierre L. de Bourgknecht ALS Research Foundation and the NINDS (5R01NS050557-02 and 5R01NS05050641-04). JTR receives support from the National Institute of Aging.


