All published articles of this journal are available on ScienceDirect.
Clinical Profile of Functional Neurological Disorder
Abstract
Introduction
Functional Neurological Disorders (FNDs) are involuntary, disabling conditions often linked to biopsychosocial factors. There is a gap in the literature regarding the clinical characteristics of patients suffering from FNDs. This study aimed to describe and compare the clinical profiles of the most common FND subtypes (seizures and motor).
Methods
This cross-sectional study included data collection from semi-structured interviews, medical records, and the Hamilton scales.
Results
Among 105 patients, 60 participants were found to be eligible. Statistically differences were found in mean age of symptom onset (seizures: 25 years vs. motor: 36 years, p<0.001), mean time to diagnosis (seizures: 14 years vs. motor: 3 years, p<0.001), symptom frequency (p=0.003), psychological violence (32% in seizures vs. 10% in motor, p=0.039), sexual trauma (76% in motor vs. 26% in seizures, p<0.001), sexual complications (41% in motor vs. 13% in seizures, p=0.013), loss of close relatives (48% in seizures vs. 10% in motor, p<0.001), and number of hours of sleep per night (p=0.009). Differences were also found in family history of epilepsy (48% in seizures vs. 21% in motor, p=0.023), movement disorders (31% in motor vs. 10% in seizures, p=0.040), and schizophrenia (31% in motor vs. 7% in seizures, p=0.016). The seizure subtype had slightly higher HAM-A (38.6) and lower HAM-D (29.5) scores compared to the motor subtype (37.3 and 30.3, respectively), with statistically significant differences in agitation, weight loss, and genitourinary and somatic symptoms (p<0.05).
Discussion
The affinities and variations between the two subgroups were correlated with the state-of-the-art literature, providing valuable insights into the theoretical and clinical understanding of FNDs.
Conclusion
Patients with FNDs exhibit a consistent clinical profile with variations according to their subtypes, allowing for the refinement of knowledge about subtype-specific characteristics.
1. INTRODUCTION
Functional Neurological Disorders (FNDs) are involuntary and disabling conditions characterized by a complex and multifactorial nature that encompasses a wide range of biopsychosocial factors [1, 2]. These disorders manifest through symptoms that are not explained by structural neurological damage or other medical conditions, often presenting a significant diagnostic and therapeutic challenge for clinicians. Symptoms frequently arise or worsen in response to emotional stressors, emphasizing the strong interplay between psychological factors and the manifestation of functional symptoms, as well as their close association with psychiatric comorbidities, such as anxiety and mood disorders [3].
Currently, FND is classified into subtypes, with Functional Movement Disorders (FMDs) and Functional Seizures (FS) being among the most prevalent in clinical practice [4]. These subtypes exhibit distinct but overlapping clinical features, requiring a nuanced understanding for accurate diagnosis and effective management. The diagnosis for both FMD and FS remains primarily clinical, based on the identification of positive signs, such as the inconsistency of symptoms over time and their incongruence with recognized patterns of organic diseases. This approach underscores the importance of clinical expertise and the use of established diagnostic criteria. Effective treatment strategies involve a multidisciplinary approach, integrating psychotherapy, psychoeducation, and physical rehabilitation, complemented by pharmacological management of coexisting psychiatric conditions when needed [1-7].
In recent years, growing evidence has supported the hypothesis that FS and FMD may represent different manifestations of a shared underlying mechanism, suggesting that they are variations within the spectrum of FND [8-12]. Despite this progress, there remain considerable gaps in the literature, particularly regarding the specific clinical characteristics that distinguish these subtypes. Such gaps hinder the development of tailored diagnostic and therapeutic interventions. In this context, the present study aims to enhance the understanding of the clinical profiles of patients with FNDs, focusing specifically on their most prevalent subtypes. By providing new evidence, this study seeks to contribute to advancements in the diagnosis, management, and overall clinical approach to these complex disorders.
2. MATERIALS AND METHODS
2.1. Study Design, Sampling, and Ethics Statement
This study employed an observational, descriptive, cross-sectional design to examine clinical and psychosocial characteristics of patients diagnosed with Functional Neurological Disorders (FNDs). Conducted in a specialized outpatient clinic, the research focused on two prevalent subtypes: Functional Movement Disorders (FMDs) and Functional Seizures (FS). Data collection included both quantitative and qualitative methods, utilizing semi-structured interviews with participants and comprehensive medical record reviews. These methods allowed for a detailed exploration of the participants' clinical histories, symptomatology, and psychosocial contexts.
The DSM-5 criteria were used as the reference for establishing the diagnosis of FND as well as its subtypes [13]. Diagnostic tests, such as the Hoover sign, among others, and examinations, including video-electroencephalography (vEEG), were employed to support the diagnostic process when necessary [1-13].
The study design adhered strictly to the STROBE guidelines for observational studies, ensuring the methodological rigor and transparency required for reliable data interpretation [14]. Ethical considerations were a priority, with the study conducted in full compliance with the principles outlined in the Declaration of Helsinki [15]. Approval was obtained from the institutional ethics committee prior to initiation, under the IRB protocol #23336819.8.0000.0096. Participants were informed of the study objectives and procedures, and written informed consent was obtained from all individuals prior to their inclusion in the research.
2.2. Eligibility and Exclusion Criteria
The eligibility criteria for participation were as follows: 1) being at least 18 years old; 2) having a documented or clinically established diagnosis of FND, confirmed by a specialized multidisciplinary team; 3) providing assent and consent to participate in the research and signing the Free and Informed Consent Form (FICF). The exclusion criteria included: 1) presence of neurological comorbidities; 2) classification under more than one subtype of FND; 3) inability to respond to the instruments due to any medical, psychological, and/or social condition; 4) patients who had been asymptomatic for at least 8 weeks; 5) refusal to sign the FICF or participants who could not be contacted to participate in the study.
Patients with neurological comorbidities were excluded to ensure a more homogeneous comparison between the two groups. This criterion aimed to include only FND cases and avoid mixed cases, such as patients with both functional seizures and epilepsy. Therefore, patients with conditions like epilepsy, movement disorders (e.g., gait disturbances and ataxias), and other organic or structural neurological diseases were not included, as these could compromise the validity of the comparisons.
2.3. Data Collection
In alignment with the eligibility criteria, researchers conducted a thorough review of the medical records of patients treated at the specialized outpatient clinic for Functional Neurological Disorders (FNDs). Following this review, patients who met the inclusion criteria were invited to participate in the study. During the recruitment process, detailed explanations about the research objectives and procedures were provided to ensure participants’ understanding and voluntary involvement. Informed consent was obtained through the signing of the Free and Informed Consent Form (FICF), which was a prerequisite for data collection.
For data collection, the researchers utilized a semi-structured assessment form developed based on the current literature on the subject, encompassing sociodemographic, clinical, objective, and subjective data, as detailed in the Supplementary Material. In addition to the questionnaire, two standardized psychometric instruments were also utilized: the Hamilton Depression Rating Scale (HAM-D) and the Hamilton Anxiety Rating Scale (HAM-A). These scales are well-established in both clinical practice and research for their reliability in assessing mood and anxiety disorders. The HAM-D, consisting of 17 items, evaluates depressive symptoms, while the HAM-A, comprising 14 items, measures anxiety levels. Although these tools provide valuable insights into the emotional states of participants, their application in this study was designed to complement clinical evaluations rather than serve as diagnostic instruments [16, 17].
All assessments were conducted directly by the researchers, ensuring consistency and accuracy in data collection. It is essential to note that no financial incentives or compensation were offered to participants, thereby emphasizing the voluntary nature of their involvement and maintaining the ethical integrity of the study.
2.4. Statistical Analysis
Quantitative variables in the study were summarized using descriptive statistics, including mean, standard deviation, minimum, and maximum values. Categorical variables, on the other hand, were characterized by their frequencies and percentages, providing a clear overview of the distribution of these data. For comparative analyses, the choice of statistical tests depended on the type and distribution of the variables. The student's t-test for independent samples was applied to normally distributed quantitative variables, while the Mann-Whitney U test, a non-parametric alternative, was used for those that did not meet the normality assumptions.
For categorical variables, Fisher’s exact test was employed, ensuring robustness and reliability even with smaller sample sizes or low-frequency categories. Statistical significance was defined as p<0.05, reflecting widely accepted standards in clinical research. To control possible errors in multiple comparisons, appropriate statistical adjustments were applied, including the Bonferroni correction for pairwise comparisons and the calculation of effect size measures. All statistical analyses were performed using SPSS Statistics software (version 30.0.0.0), which provided a comprehensive platform for data management and interpretation, ensuring the methodological rigor and accuracy of the findings.
The sample size was determined based on a review of the existing literature on Functional Neurological Disorders (FNDs), ensuring alignment with prior studies in the field. Additionally, statistical analyses were conducted to confirm the adequacy of the sample size. A power analysis was performed to ensure sufficient statistical power, and effect size measures were used to assess the magnitude of group differences. These approaches helped validate the reliability of our findings and the robustness of the statistical comparisons.
3. RESULTS
3.1. Data Analysis
Data collection was conducted between January 2020 and May 2022, spanning a total period of 28 months. During this time, 105 medical records from patients treated at the specialized outpatient clinic were thoroughly reviewed to identify eligible participants. Of these, 60 individuals met the inclusion criteria and consented to participate in the study. The sample comprised 31 patients diagnosed with the seizure subtype and 29 with the motor subtype, reflecting the prevalence of these two primary subtypes in clinical practice. This detailed review process ensured a robust sample that accurately represents the clinical diversity observed within Functional Neurological Disorders (Fig. 1).
3.2. Clinical Profile
Table 1 presents data on the clinical profile of the participants, stratified by FND subtype. Statistically significant differences were observed in the following variables: age of symptom onset, time to receive FND diagnosis (in years), symptom frequency, history of violence, family history of illness, sleep, sexual health, and the loss of a close relative.
| - | Seizures, n = 31 | Motor, n = 29 | x2 / t* | p |
|---|---|---|---|---|
| Gender (F:M**) | 22:9 | 21:8 | 0.015 | 0.901 |
| Age of onset of symptoms, mean [SD†] | 25 [8] | 36 [10] | -4.821 | <.001‡ |
| Years to receive diagnosis, mean [SD] | 14 [9] | 3 [2] | 6.686 | <.001‡ |
| Symptom frequency | ||||
| Daily, n (%) | 8 (26) | 18 (62) | 14.129 | 0.003‡ |
| 3 times a week, n (%) | 12 (39) | 4 (14) | ||
| 1 time a week, n (%) | 5 (16) | 7 (24) | ||
| Once every 15 days, n (%) | 6 (19) | - | ||
| Potentially triggering factors | ||||
| Anxiogenic situations, n (%) | 28 (90) | 12 (42) | 0.742 | 0.316 |
| Unmotivated, n (%) | 3 (10) | 5 (17) | ||
| Mood most of the day | ||||
| Euthymic, n (%) | 8 (26) | 7 (24) | 2.332 | 0.506 |
| Hyperthymic, n (%) | 6 (19) | 2 (7) | ||
| Labile, n (%) | 6 (19) | 8 (28) | ||
| Hypothymic, n (%) | 11 (36) | 12 (41) | ||
| Use of psychoactive substances | ||||
| Alcohol, n (%) | - | 3 (10) | 3.376 | 0.066 |
| Tabaco, n (%) | 4 (13) | 2 (7) | 0.601 | 0.438 |
| Illicit Substances, n (%) | - | - | - | - |
| Psychiatric events | ||||
| Suicide attempts, n (%) | 16 (52) | 17 (59) | 0.297 | 0.586 |
| Sensory-perceptual changes, n (%) | 12 (39) | 10 (37) | 0.115 | 0.734 |
| Delusions, n (%) | 10 (32) | 9 (31) | 0.01 | 0.919 |
| Risk behaviors, n (%) | 8 (26) | 12 (41) | 1.635 | 0.158 |
| History of Violence | ||||
| Physical, n (%) | 14 (45) | 13 (45) | 0.001 | 0.979 |
| Phychological, n (%) | 10 (32) | 3 (10) | 4.239 | 0.039‡ |
| Sexual, n (%) | 8 (26) | 22 (76) | 15.017 | <.001‡ |
| Family history of illness | ||||
| Epilepsy, n (%) | 15 (48) | 6 (21) | 5.053 | 0.023‡ |
| Movement disorders, n (%) | 3 (10) | 9 (31) | 4.271 | 0.040‡ |
| Mood disorders, n (%) | 12 (39) | 10 (36) | 0.115 | 0.734 |
| Anxiety disorders, n (%) | 10 (32) | 10 (35) | 0.033 | 0.855 |
| Substance Abuse, n (%) | 4 (13) | 5 (17) | 0.221 | 0.638 |
| Schizophrenia, n (%) | 2 (7) | 9 (31) | 6.048 | 0.016‡ |
| Number of hours of sleep per night | ||||
| Less than 6 hours, n (%) | 4 (13) | 9 (31) | 11.499 | 0.009‡ |
| 7 to 9 hours, n (%) | 17 (55) | 4 (14) | ||
| 10 to 11 hours, n (%) | 6 (19) | 11 (38) | ||
| 12 hours or more, n (%) | 4 (13) | 5 (17) | ||
| Sleep disorders, n (%) | 20 (66) | 19 (66) | 0.007 | 0,935 |
| Libido | ||||
| Preserved, n (%) | 9 (29) | 4 (14) | 5.211 | 0.049‡ |
| Increased, n (%) | 3 (10) | - | ||
| Decreased, n (%) | 19 (61) | 25 (86) | ||
| Sexual satisfaction | ||||
| Absent, n (%) | 18 (58) | 24 (83) | 4.351 | 0.035‡ |
| Preserved, n (%) | 13 (42) | 5 (17) | ||
| Sexual dysfunctions, n (%) | 4 (13) | 12 (41) | 6.213 | 0.013‡ |
| Assistance with ADLs|| and IADLs¶ | ||||
| Family member, n (%) | 27 (87) | 25 (86) | 0.016 | 0.850 |
| Formal caregiver, n (%) | 2 (6) | 2 (7) | ||
| Do not require, n (%) | 2 (6) | 2 (7) | ||
| Dysfunctional family, n (%) | 16 (52) | 19 (66) | 1.192 | 0.275 |
| Loss of close relative, n (%) | 15 (48) | 3 (10) | 10.326 | <.001‡ |
| Other traumatic events§, n (%) | 10 (32) | 12 (41) | 0.537 | 0.464 |
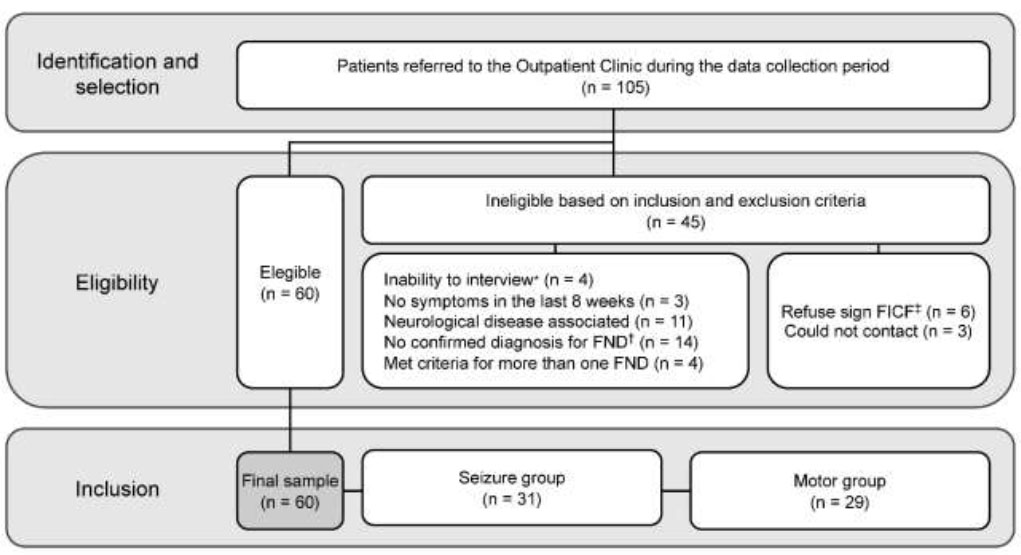
Stratification of the study sample. Note: *Inability to respond to the instruments due to any medical, psychological, and/or social condition; FND, †Functional Neurological Disorder; ‡FICF, Free and Informed Consent Form.
3.3. Hamilton Scales
As shown in Table 2, on the HAM-A scale, the seizure subtype had an average score of 38.6 compared to 37.3 for the motor subtype. On the HAM-D scale, the seizure subtype averaged 29.5, while the motor subtype scored slightly higher at 30.3 points. Statistically significant differences were observed in the genitourinary symptoms on the HAM-A scale, as well as in the variables of agitation, somatic symptoms (including gastrointestinal symptoms), genital symptoms, and weight loss on the HAM-D scale.
| - | Seizures, n = 31 | Motor, n = 29 | - | - |
|---|---|---|---|---|
| Mean [SD*] | t† | p | ||
| Hamilton Anxiety Rating Scale | ||||
| Anxious mood | 3.1 [0.9] | 3.1 [1.1] | -0.016 | 0.493 |
| Tension | 3.2 [1.2] | 3.0 [1.2] | 0.726 | 0.235 |
| Fears | 3.0 [1.4] | 2.4 [1.7] | 1.576 | 0.060 |
| Insomnia | 2.0 [1.8] | 2.2 [1.6] | -0.399 | 0.346 |
| Difficulties in concentration and memory | 3.0 [0.9] | 2.7 [1.2] | 0.503 | 0.309 |
| Depressed mood | 3.0 [1.2] | 3.1 [1.1] | -0.474 | 0.318 |
| General somatic symptoms: muscular | 3.1 [1.0] | 3.3 [0.8] | -0.918 | 0.181 |
| General somatic symptoms: sensory | 2.9 [1.1] | 2.9 [1.2] | 0.015 | 0.494 |
| Cardiovascular symptoms | 3.1 [0.9] | 2.7 [1.2] | 1.459 | 0.075 |
| Respiratory symptoms | 2.6 [1.4] | 2.2 [1.4] | 0.837 | 0.203 |
| Gastrointestinal symptoms | 2.6 [1.4] | 2.7 [1.2] | -0.421 | 0.338 |
| Genito-urinary symptoms | 2.4 [1.3] | 2.9 [1.0] | -1.745 | 0.043‡ |
| Other autonomic symptoms | 3.2 [0.9] | 2.9 [1.2] | 1.166 | 0.124 |
| Behavior during the interview | 1.6 [1.2] | 1.2 [1.3] | 1.169 | 0.123 |
| Total | 38.6 [9.0] | 37.3 [11.0] | 0.47 | 0.320 |
| Hamilton Depression Rating Scale | ||||
| Depressed mood | 2.4 [1.4] | 2.4 [1.1] | 0.024 | 0.490 |
| Feelings of guilt | 2.3 [1.1] | 2.1 [1.2] | 0.419 | 0.338 |
| Suicide | 3.1 [1.3] | 2.5 [1.6] | 1.509 | 0.068 |
| Insomnia-early | 1.1 [0.9] | 1.2 [0.9] | -0.602 | 0.275 |
| Insomnia-middle | 0.9 [0.8] | 1.0 [0.9] | -0.468 | 0.321 |
| Insomnia-late | 0.8 [0.8] | 1.1 [0.9] | -1.503 | 0.069 |
| Work and activities | 3.9 [0.3] | 3.9 [0.3] | -0.766 | 0.223 |
| Retardation | 0.9 [1.0] | 1.0 [1.0] | -0.653 | 0.258 |
| Agitation | 0.9 [0.8] | 1.8 [1.1] | -3.392 | <0.001‡ |
| Anxiety | 2.6 [1.0] | 2.8 [1.0] | -0.865 | 0.195 |
| Anxiety – somatic | 3.3 [0.9] | 3.1 [1.0] | -0.514 | 0.304 |
| Somatic symptoms (gastrointestinal) | 1.3 [0.8] | 0.8 [0.8] | 2.281 | 0.013‡ |
| Somatic symptoms | 1.6 [0.8] | 1.8 [0.4] | -1.306 | 0.098 |
| Genital symptoms | 1.0 [0.8] | 1.8 [0.4] | -4.445 | <0.001‡ |
| Hypochondriasis | 2.3 [1.1] | 2.2 [1.1] | 0.179 | 0.429 |
| Weight loss | 1.2 [1.5] | 0.6 [0.9] | 1.972 | 0.027‡ |
| Insight | 0.4 [0.7] | 0.4 [0.6] | -0.166 | 0.434 |
| Total | 29.5 [5.9] | 30.3 [7.0] | -0.474 | 0.319 |
4. DISCUSSION
4.1. Clinical Profile
An association was observed between patients with the seizure subtype and an earlier onset of symptoms, as well as a longer time to receive an accurate diagnosis compared to those with the motor subtype. These findings are consistent with the literature, which emphasizes the diagnostic complexity of FNDs, particularly in seizure subtype cases, which are often misdiagnosed as other neurological conditions and require advanced diagnostic tests, such as video-EEG, for confirmation [5, 18-20]. This misdiagnosis tends to lead to unfavorable prognoses and prolonged inadequate management, such as the use of antiepileptic medications due to confusion with epileptic neurological conditions, negatively impacting quality of life and contributing to the chronicity of the functional condition [1, 2, 5, 8, 9, 19, 20].
The observed differences in symptom frequency and intensity are likely associated with the broad spectrum of functional manifestations of FND. These variations not only highlight the heterogeneity of the disorder but also serve as critical factors in differentiating its subtypes [2, 4, 21]. For example, individuals with the seizure subtype often experience symptoms with abrupt onset and fluctuating severity, whereas those with the motor subtype may demonstrate more persistent and physically debilitating manifestations [2, 4, 21]. This symptom diversity underscores the importance of tailored diagnostic and therapeutic approaches, as the nuances in symptomatology provide valuable insights into the underlying mechanisms driving each subtype [1-6]. As such, understanding these differences is crucial for refining diagnostic criteria, enhancing clinical management, and maximizing patient outcomes [2, 4, 21].
Regarding situations that trigger symptoms, participants with the seizure subtype tend to experience a greater variety of factors, with episodes often associated with anxiety-inducing situations. In contrast, participants with the motor subtype were associated with more consistent symptoms, even in the absence of a clear trigger. This pattern suggests that patients with the seizure subtype are more sensitive to conflicts and external stimuli, showing heightened emotional reactivity to stress, whereas the predominant labile and dysthymic mood in patients with the motor subtype indicates a chronic and internalized basis for the formation and maintenance of motor symptoms. These findings align with previous studies, which indicate that patients with the seizure subtype tend to have slightly higher levels of anxiety compared to those with the motor subtype [7-9, 22].
With regard to psychiatric events, our findings suggest a strikingly high prevalence of these factors across both FND subtypes. This observation may highlight a shared vulnerability among patients, irrespective of their specific subtype. Both groups were associated with elevated rates of suicide attempts, perceptual disturbances, delusions, and risk behaviors. Such manifestations are likely interconnected with the substantial burden of psychiatric comorbidities, including mood and anxiety disorders, which are commonly associated with FND [1, 4, 22]. These findings underscore the profound impact of mental health disorders on the clinical course of FND, suggesting that addressing these comorbidities is critical for effective management [1, 4, 22]. Moreover, the similarity in the prevalence of psychiatric events across subtypes reinforces the need for comprehensive psychological assessments in this population, as these factors can significantly influence symptom chronicity, treatment outcomes, and overall quality of life [1, 4, 22].
We also identified associations between a history of violence and the FND subtypes. Psychological abuse was more associated with the seizure subtype, while sexual abuse was more associated with the motor subtype group. These findings suggest that precipitating factors may be associated with the development of the FND subtype and emphasize the importance of a thorough investigation of the patient's clinical history. These results are consistent with studies highlighting the link between past trauma and the development of functional symptoms [20, 23], suggesting that life history and adverse experiences play a crucial role in the formulation and manifestation of FND.
Regarding other adverse life events, such as illnesses, accidents, and surgeries, our findings suggest an association between these events and FND. These experiences may not only impact physical health but also contribute to the psychological burden that exacerbates FND symptoms, with previous studies demonstrating a significant association between increased emotional vulnerability and the practical consequences of dealing with chronic illnesses or accidents, along with the physical and psychological strain of coping with these situations [20, 23]. This heightened vulnerability may act as a catalyst, contributing to the onset, worsening, and chronicity of functional symptoms, as the emotional distress associated with these adverse events could lead to maladaptive coping mechanisms, further complicating the clinical course of FND [20, 23].
An analysis of the family history of illness revealed an association between the seizure subtype and a higher prevalence of epilepsy in family members, while the motor subtype was more associated with movement disorders and schizophrenia. These findings suggest that family history may play a critical role in the emergence of FND subtypes, indicating a potential genetic or environmental predisposition, as current literature states that family dynamics may significantly influence the development of functional symptoms, with exposure to familial patterns of illness and behavior potentially affecting the patient’s response to stress and health issues [8, 24-26]. Additionally, modeling and learning from family members, as well as the potential for secondary gains, such as attention, support, or avoidance of responsibilities, could contribute to the formation and maintenance of functional symptoms [8, 24-26].
Sleep-related findings revealed an association between the seizure subtype and shorter sleep duration compared to the motor subtype. However, both groups were associated with poor sleep quality and a high prevalence of sleep disorders. Poor sleep is often seen as both a consequence and a contributor to the persistence of functional symptoms, creating a vicious cycle that affects overall well-being, as it may be associated with the heightened anxiety, emotional distress, or physical discomfort associated with the disorder, which, in turn, can affect the body’s ability to rest and recover [27, 28].
In relation to sexual health, although the impacts are associated with both subtypes, our results tend to indicate that patients with the motor subtype experience greater impairments, including reduced sexual desire, sexual dysfunctions, and lack of sexual satisfaction, more prominently compared to patients with the seizure subtype. Sexual dysfunctions are common in patients with neurological disorders and are often exacerbated by the presence of persistent motor symptoms that negatively affect self-image and self-esteem [29, 30].
A notable association may exist between a history of sexual abuse and the sexual impairments observed in patients with the motor subtype. More than three-quarters of these patients report experiencing sexual abuse during childhood or adolescence, suggesting an association with more pronounced sexual dysfunction and difficulties in sexual expression within this group. The interplay of these factors may create a feedback loop, where sexual dysfunction increases emotional distress, thereby exacerbating functional symptoms, which, in turn, accentuate the difficulty in expressing sexuality, as the impact of sexual trauma on individuals' mental and sexual health is well-documented, with evidence linking traumatic sexual experiences to mental illness, sexual dysfunction, and difficulties in intimate relationships [31, 32].
The need for assistance with ADLs and IADLs was observed in both groups, suggesting an association between functional symptoms and reduced autonomy. This dependency may also influence family dynamics, as family members often take on caregiving roles. Prolonged caregiving can impair caregivers' quality of life, leading to emotional burden, physical exhaustion, mental health challenges, deteriorating relationships, and exacerbating dysfunctional family dynamics, which may also be associated with the high rates of family conflicts observed in both subtypes [33, 34].
Additionally, the finding that the death of a close family member was significantly more prevalent in the seizure subtype suggests that the loss of a significant figure may be associated with the onset and/or exacerbation of functional symptoms. The loss of key relationships can amplify feelings of abandonment, insecurity, and helplessness, potentially leading to the somatization of internal conflicts, which may manifest as functional symptoms [23, 35-37].
4.2. HAM-A and HAM-D Scales
The similarity in scores across both subtypes tends to suggest a shared profile characterized by severe anxiety and depressive disorders, consistent with previous studies that emphasize the high prevalence of psychiatric comorbidities in FND [1-5, 8, 10, 19, 22, 38]. These findings, in line with current literature, suggest that mood disorders may contribute to chronicity and exacerbation of symptoms, while anxiety disorders may be involved in triggering and maintaining functional symptoms, impacting clinical management and treatment response [1-5, 8, 10, 19, 22, 38].
Subtle differences emerged between subtypes; the seizure subtype was more associated with higher scores on items related to anxious mood, insomnia, and guilt. In contrast, the motor subtype was more associated with higher scores for somatic anxiety, including gastrointestinal discomfort and physical pain. These nuances suggest that while both subtypes share high levels of anxiety and depression, the symptomatology of each subtype may be associated with different forms of body perception and emotional response, potentially serving as etiological markers for the differentiation of FND [39-41].
5. LIMITATIONS
The small sample size in this study may limit the generalizability of the findings, particularly when attempting to compare different subtypes of Functional Neurological Disorders (FNDs). A larger and more diverse sample would provide a more comprehensive understanding of the various clinical manifestations and outcomes across the FND spectrum. Additionally, the reliance on data from medical records and semi-structured interviews introduces the possibility of reporting biases, which could affect the accuracy and consistency of the information collected. Participants may not always provide fully accurate or consistent reports, and medical records may contain incomplete or biased data. To mitigate these limitations, data collection was standardized through the use of a structured assessment form, and all interviews were conducted by trained researchers to ensure consistency and reduce subjective variability. Furthermore, whenever possible, self-reported information was cross-verified with medical records to enhance data reliability. However, despite these measures, some degree of reporting bias remains inherent to the study design. The cross-sectional nature of the study also prevents the establishment of causality between the identified factors and the onset or progression of FND symptoms. This limitation underscores the importance of conducting longitudinal studies, which would allow for the exploration of temporal relationships and provide a clearer understanding of the causal mechanisms underlying these disorders. Finally, the fact that data were collected from a single clinical center limits the external validity of the results. This geographic and institutional restriction may hinder the broader applicability of the findings to other populations or clinical settings. To enhance the generalizability of future research, multicenter studies involving larger, more diverse populations are needed to validate these findings and explore regional or cultural differences in the presentation and management of FNDs.
CONCLUSION
Patients with FNDs are associated with a consistent clinical profile that presents variations according to their subtype. The differences observed between FND subtypes have important clinical implications, particularly in diagnosis and treatment approaches. The earlier onset and prolonged time to diagnosis in the seizure subtype highlight the need for improved recognition of functional seizures to prevent unnecessary treatments, such as antiepileptic medications, which may contribute to chronicity and reduced quality of life. The association between the motor subtype and persistent symptoms, somatic anxiety, and a history of sexual trauma suggests that treatment strategies should consider trauma-informed care and multidisciplinary rehabilitation approaches. Furthermore, the high prevalence of psychiatric comorbidities in both subtypes reinforces the necessity of integrating mental health support into FND management, emphasizing that treatment must be multidisciplinary. Addressing sleep disturbances and sexual health concerns, which were differentially associated with each subtype, may further enhance patient outcomes by targeting symptom-specific burdens. These findings support a more tailored approach to FND treatment, where subtype-specific characteristics inform personalized therapeutic strategies aimed at improving prognosis and quality of life. Given the significant impact of functional symptoms on autonomy and daily functioning, the role of family members and caregivers should be carefully considered in treatment planning. Clinicians should consider integrating psychoeducation and structured support for caregivers to help mitigate stress-related factors that may reinforce symptom chronicity. Future research, including longitudinal and multicenter studies, may help validate and expand upon our findings.
AUTHORS’ CONTRIBUTIONS
The authors confirm contribution to the paper as follows: B.G., D.P.: Writing, reviewing, evaluation, editiong, interpretation, data analysis; H.A.G.T.: Writing, reviewing and editing; M.D.H.: Data analysis and interpretation. All authors have read and agreed to the published version of the manuscript.
LIST OF ABBREVIATIONS
| FNDs | = Functional Neurological Disorders |
| FS | = Functional Seizures |
| FMDs | = Functional Movement Disorders |
| DSM-5 | = Diagnostic and Statistical Manual of Mental Disorders Fifth Edition |
| vEEG | = Video-Electroencephalography |
| F | = Female |
| M | = Male |
| HAM-D | = Hamilton Depression Rating Scale |
| HAM-A | = Hamilton Anxiety Rating Scale |
| ADLs | = Activities of Daily Living |
| IADLs | = Instrumental Activities of Daily Living |
| FICF | = Free and Informed Consent Form |
| SPSS | = Statistical Package for the Social Sciences |
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
Approval was obtained from the institutional ethics committee of the Clinical Hospital of the Federal University of Paraná prior to initiation, under the IRB protocol #23336819.8.0000.0096.
HUMAN AND ANIMAL RIGHTS
All human research procedures followed were in accordance with the ethical standards of the committee responsible for human experimentation (institutional and national), and with the Helsinki Declaration of 1975, as revised in 2013.
CONSENT FOR PUBLICATION
Participants were informed of the study objectives and procedures, and written informed consent was obtained from all individuals prior to their inclusion in the research.
AVAILABILITY OF DATA AND MATERIALS
The data and supportive information are available within the article.
ACKNOWLEDGEMENTS
Declared none.


