All published articles of this journal are available on ScienceDirect.
Thrombocytosis in Hemorrhagic Stroke: Case Report and Literature Review
Abstract
Background
Thrombocytosis is a condition characterized by a platelet count higher than 450,000/μl. In instances of severe thrombocytosis, the number of platelets reaches 1,000,000 per microliter. Thrombocytosis is commonly identified as an unexpected aberration in laboratory tests, as the majority of patients do not show any symptoms. Thrombocytosis can be categorized into two types: essential thrombocytosis (primary) and reactive thrombocytosis (secondary). Reactive thrombocytosis is the predominant form of thrombocytosis. Within the population of persons with thrombocytosis, around 80% to 90% are specifically identified as having reactive thrombocytosis.
Case Presentation
This is a case report of a 73-year-old man who arrived at the emergency room with symptoms, including loss of consciousness, weakness on the right side of his body, high blood pressure, and difficulty breathing. Further investigations have revealed the patient to have a high platelet count (1,186,000/µL), a hemorrhagic stroke, and pneumonia.
Discussion
This case report describes the presence of thrombocytosis in a patient who has experienced a hemorrhagic stroke. The patient displayed a condition of extreme thrombocytosis, marked by a platelet count exceeding 1,000,000 per microliter (µL). The brain CT scan showed a 42 cc intracerebral haemorrhage in the right temporal lobe, resulting in impending obstructive hydrocephalus. The patient also reported experiencing dyspnoea and fever one week prior to hospitalisation, and his sputum culture revealed the presence of Klebsiella pneumoniae bacteria.
Conclusion
This case report demonstrates the clinical presentation of a hemorrhagic stroke accompanied by reactive thrombocytosis, with pneumonia being the major infection linked with reactive thrombocytosis.
1. INTRODUCTION
Thrombocytosis, also known as thrombocythemia, is a condition characterised by an abnormally high platelet count, which is defined as exceeding 450,000 platelets per microliter (μl) of blood. In instances of severe thrombo- cytosis, the number of platelets reaches 1,000,000 per microliter (µL). Thrombocytosis is commonly identified as an unexpected aberration in laboratory tests, as the majority of patients do not show any symptoms. Thrombo- cytosis can be categorised into two types: essential thrombocytosis (primary) and reactive thrombocytosis (secondary) [1-3]. This differentiation between primary and secondary thrombocytosis is significant because it has consequences for assessment, prognosis, and treatment [2].
Essential thrombocytosis is caused by the uncontrolled abnormality in the production of platelets by bone marrow progenitor cells and is commonly linked to Myelopro- liferative Neoplasms (MPN) [2]. Essential thrombocytosis is characterised by an excessive growth of mega- karyocytes in the bone marrow [4]. In the majority of cases, primary thrombocytosis is associated with genetic disorders [1, 4].
The World Health Organisation (2016) states that essential thrombocytosis can be diagnosed when the platelet count exceeds 450,000/μl; a bone marrow biopsy demonstrates the proliferation of the megakaryocyte lineage with an increase in enlarged mature mega- karyocytes with hyperlobulated nuclei and a minor (grade 1) increase in reticulin fibres, and there is either a mutation in the oncogene of the myeloproliferative leukaemia virus (JAK2), Calreticulin (CALR), or Janus Kinase 2 (JAK2), or the Myeloproliferative Leukaemia virus (MPL); these conditions do not meet the WHO criteria for CML, PV, PMF, MDS, or other myeloid neoplasms, and lack clonal or reactive causes (Table 1) [1, 4]. The JAK2 mutation is present in about 55% of patients with essential thrombocytosis [4].
|
Major criteria • Platelet count of >450,000/μL. • Bone marrow biopsy showing proliferation of the megakaryocyte lineage with increased numbers of enlarged mature megakaryocytes with hyperlobulated nuclei and minor (grade 1) increase in reticulin fibers. • Not meeting WHO criteria for CML, PV, PMF, MDS, or other myeloid neoplasms. • JAK2, CALR, or MPL mutations. |
|
Minor criteria • The presence of a clonal marker or absence of reactive thrombocytosis. • Diagnosis requires all four major or first three major and one minor criteria. |
Reactive thrombocytosis refers to an elevated number of platelets in the blood, which occurs without the presence of chronic myeloproliferative illness. Instead, it is caused by an underlying event, such as infection, inflammation, haemorrhage, certain diseases, or specific drugs. Reactive thrombocytosis is the predominant form of thrombocytosis and is typically detected with regular laboratory tests. The majority of persons with thrombo- cytosis, specifically 80% to 90%, are diagnosed with reactive thrombocytosis. Typically, symptoms of reactive thrombocytosis arise from an underlying illness rather than the thrombocytosis itself. Reactive thrombocytosis is a laboratory abnormality that goes away when the underlying cause is resolved [2]. In a study involving 280 patients with extremely high platelet count (≥1,000,000/μL), 82% (n=231) had reactive causes of thrombocytosis, 4% (n=11) had unknown causes, and only 14% (n=38) had a disorder that caused elevated platelet count [5]. Some of the causes of reactive thrombocytosis are listed in Table 2.
| Infection Tissue damage Inflammation Malignancy Acute bleeding, hemolytic anemia Rebound after chemotherapy-induced thrombocytopenia Rebound after vitamin B12 correction in pernicious anemia Iron deficiency Medications: aztreonam, ceftazidime, ibuprofen, epinephrine, glucocorticoids Trauma, major surgery, post-operative complications Response to exercise Post-splenectomy or asplenia (congenital or functional) |
In adults, reactive thrombocytosis is mostly caused by infection, tissue injury, chronic inflammatory diseases, or cancer. These factors are present in over 75% of instances of reactive thrombocytosis [6].
2. CASE PRESENTATION
A 73-year-old male patient presented to the emergency department at Adam Malik Hospital, located in Medan, North Sumatra, Indonesia, exhibiting a state of uncons- ciousness. According to a witness, he experienced a quick commencement of changes in his mental state and weakness on the right side of his body. The family reported that the patient had been diagnosed with hypertension for a duration of 10 years, but exhibited poor adherence to his prescribed antihypertensive treatment. There was no record of the individual having diabetes mellitus or heart disease. The patient reported expe- riencing dyspnoea and fever one week before the hospitalisation, which then increased during the course of the three days, leading up to admission. The patient had also reported experiencing coughing for one month before the admission.
Upon admission, the patient exhibited a state of drowsiness and a neurological examination revealed left-sided dominance, as indicated by a National Institutes of Health Stroke Scale score of 17. The lungs were examined and bilateral rhonchi were detected. The patient's vital signs were recorded as follows: blood pressure of 190/100 mmHg, heart rate of 89 beats per minute, respiratory rate of 20 breaths per minute, body temperature of 36.5oC, and SpO2 of 92% in room air. The initial laboratory workup in the emergency department revealed the following results: White Blood Cell (WBC) count of 46,430 per microliter; haemoglobin level of 12.9 grams per decilitre; red blood cell count of 4.37 million per microliter; haematocrit level of 37.7%; platelet count of 1,186,000 per microliter; mean corpuscular volume of 86 femtoliters; basic metabolic panel showing a creatinine level of 2.51 milligrams per decilitre and a normal glucose level of 168 grams per decilitre; procalcitonin level of 2.3 nanograms per millilitre. The coagulation investigations yielded the following results: prothrombin time of 13.4 seconds, partial thromboplastin time of 38.8 seconds, and an international normalised ratio of 1.23. The study on arterial blood gas revealed the presence of respiratory alkalosis. A subsequent study on peripheral blood morphology revealed normocytic-normochromic red blood cells, elevated leukocyte count, and increased platelet count with macro platelets. The patient was diagnosed with reactive thrombocytosis.
The brain CT scan showed a 42 cc intracerebral haemorrhage in the right temporal lobe, which caused a subfalcine herniation of approximately 0.8 mm to the left side. This also resulted in impending obstructive hydro- cephalus. Additionally, there was a subarachnoid haemor- rhage in the sulci of the left parietal lobe, and an intraventricular haemorrhage in the posterior horn of both lateral ventricles, as depicted in Fig. (1). The patient was recommended to undergo decompressive craniectomy, but the family refused to proceed with the surgical procedure. The patient was treated in an Intensive Care Unit (ICU) to address issues related to the airway, blood pressure, and Intracranial Pressure (ICP).
The patient's prescribed drugs included a mannitol infusion, nicardipine, ceftriaxone injection of 2g adminis- tered twice daily, candesartan of 8 mg taken daily, and atorvastatin of 20 mg taken once daily. At first, the management prioritised the cautious lowering of intracranial pressure and regulation of blood pressure, as well as the treatment of pneumonia infection. The sputum culture revealed the presence of Klebsiella pneumoniae bacterium, and the resistance test indicated that it was resistant to ceftriaxone, but sensitive to meropenem. According to these findings, the antibiotic was switched to meropenem injection, with a dosage of 1 gram administered three times a day. Platelet count was evaluated with regular blood testing. By the third day of being hospitalised, the patient's platelet count dropped to 943,000 per microliter (µL). During the eighth and sixteenth days of hospitalisation, the platelet count dropped to 815,000/µL and 699,000/µL, respectively. The patient's duration of stay in the Intensive Care Unit (ICU) was 11 days before being transferred to a standard inpatient room.
3. DISCUSSION
This case presentation outlines the occurrence of thrombocytosis in a patient with hemorrhagic stroke. The patient exhibited severe thrombocytosis, characterised by a platelet count exceeding 1,000,000/µL. Thrombocytosis is commonly identified as an unexpected anomaly during laboratory testing. In this case, thrombocytosis was discovered through standard laboratory evaluation in the emergency room. Typically, the majority of cases, ranging from 80% to 90%, exhibit reactive thrombocytosis as a result of specific underlying disorders. In addition to experiencing a hemorrhagic stroke, the patient presented with leucocytosis, desaturation, and a medical history of dyspnea and coughing. Consequently, there was sufficient evidence to substantiate the presence of reactive thrombocytosis. The main aetiologies of thrombocytosis were excluded. In this instance, pneumonia or hemor- rhagic stroke were the presumed underlying diseases responsible for the thrombocytosis.
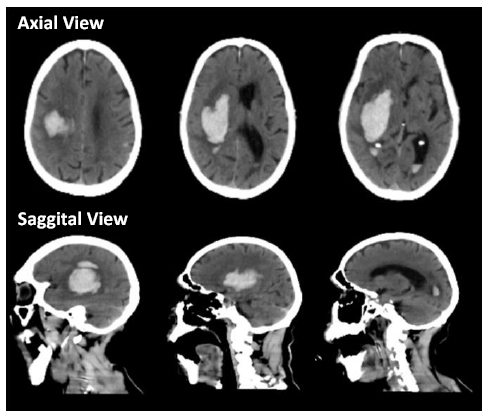
CT obtained upon arrival revealing Intracerebral Hemorrhage (ICH) in the right temporal lobe, causing subfalcine herniation to the left side by ± 0.8 mm and impending obstructive hydrocephalus; Subarachnoid Hemorrhage (SAH) could be seen to fill in the sulci of the left parietal lobe, and Intraventricular Hemorrhage (IVH) in the posterior horn of bilateral lateral ventricle.
In adults, reactive thrombocytosis is most commonly caused by infection, tissue damage, chronic inflammatory disorders, or malignancy. These factors are present in over 75% of cases [6, 7]. Among these, acute infections, particularly respiratory tract infections, like pneumonia, are the most frequently reported cause [8, 9]. Pneumonia is the main infection associated with reactive thrombo- cytosis. Thrombocytosis, which is often seen as a normal inflammatory response to infections, has recently been linked to higher mortality in hospitalised patients with Community-acquired Pneumonia (CAP). Thrombocytosis in patients with Community-acquired Pneumonia (CAP) is also linked to unfavourable prognosis, complex pleural effusion, and empyema [10, 11].
Reactive thrombocytosis occurs when there is an increase in the creation of platelets by megakaryocytes, which is triggered by the body's normal response to higher levels of thrombopoietic and inflammatory cytokines in the bloodstream. Patients with reactive thrombocytosis show increased amounts of Interleukin-1 (IL-1), IL3, IL-4, IL-6, and Tumour Necrosis Factor-α (TNF-α) in their blood. On the other hand, individuals with essential thrombocytosis have been found to have normal levels of these cytokines. Interleukin-6 is the main and consistently high cytokine in reactive thrombocytosis [12]. Platelet sequestration in the lung has been observed during the phase of Acute Lung Injury (ALI)/Acute Respiratory Distress Syndrome (ARDS), and high levels of IL-6 in the alveolar airspace indicate a strong pro-inflammatory cytokine response to traumatic injury [12, 13].
The patient in this case report suffered from acute haemorrhagic stroke, most probably attributed to uncontrolled chronic hypertension (a significant and inde- pendent risk factor for hemorrhagic stroke). Worldwide, uncontrolled hypertension accounts for approximately 75% of the risk for hemorrhagic stroke [14-16]. Simultaneously, acute bleeding is a factor that might lead to reactive thrombocytosis. Hemorrhagic stroke, defined by the rupture of blood vessels causing intracranial bleeding, initiates a complex pathophysiological reaction that may lead to reactive thrombocytosis. The bleeding causes tissue damage and inflammation, leading to the production of pro-inflammatory cytokines. These mediators activate the bone marrow to enhance platelet production as a component of the body's compensating response to the perceived hemorrhagic condition [5, 6, 17].
Conversely, thrombocytosis can also lead to acute bleeding, which is a common complication [1, 3]. This complication is more frequently associated with essential thrombocytosis rather than reactive thrombocytosis [18]. Qualitative abnormalities of platelets, including morpho- logic, functional, and metabolic changes, have been documented in patients with essential thrombocytosis and other myeloproliferative disorders. These defects are likely significant contributors to the pathogenesis of bleeding related to these conditions. The most characteristic defect is the complete absence of platelet aggregability due to the loss of platelet alpha-adrenergic receptors [19].
There is no targeted treatment for reactive thrombo- cytosis. Identification of reactive disorders is essential since thrombocytosis can be resolved by treating the underlying cause. Thus, the focus of treatment for reactive thrombocytosis is managing the underlying condition in order to normalise platelet levels. In cases of reactive thrombocytosis accompanied by infection, the focus of treatment should be on addressing the infection itself rather than solely on reducing the platelet count [2, 6]. In this particular case, the patient was initially administered an empirical antibiotic injection containing ceftriaxone. Subsequently, following the outcomes of the culture and sensitivity test, the antibiotic was changed to meropenem. During the hospital's follow-up, there was a progressive decline in the patient's platelet count, which corresponded to the improvement in the infection condition. The platelet count decreased progressively from 1,186,000/µL upon initial admission to 943,000/µL on the third day of hospitalisation, and subsequently to 815,000/µL and 699,000/µL on the eighth and sixteenth days of hospitalisation, respectively.
Reactive thrombocytosis may serve as an indicator of unfavourable prognosis. A study involving 421 individuals who received treatment for acute infections found 32 patients (7.6%) to have a mean platelet count of 527,000/µL. The study revealed that patients with thrombocytosis experienced longer hospital stays, higher incidence of bacteremia, and elevated mortality rates. Specifically, the mortality rate was 6.25% in the group with reactive thrombocytosis, compared to 2% in the group without thrombocytosis [7]. This heightened risk of mortality associated with thrombocytosis has also been observed in other studies. A cohort study investigating the correlation between platelet count upon acute hospitali- sation and mortality revealed that patients with platelet count exceeding 500,000//µL had a 2.5-fold higher risk of mortality within 30 days compared to patients with normal platelet count [20]. Additionally, another study demons- trated that an abnormal increase in platelet count in elderly patients can independently predict mortality. A study was conducted on a large group of elderly adults who were not showing any symptoms. The study aimed to investigate the relationship between platelet count and the likelihood of death. During a median follow-up period of 3.3 years, a total of 134,132 patient-years were observed. It was discovered that both thrombocytosis and thrombo- cytopenia were significantly associated with higher mortality rates in these patients. The Hazard Ratio (HR) for thrombocytosis was determined to be 1.75 [21].
Patients who later developed thrombocytosis had a higher predicted risk of death upon admission and experienced more organ failures during their time in the intensive care unit compared to patients without thrombocytosis [22]. In the case of the patient being discussed, he was an elderly individual (73 years old) with reactive thrombocytosis exceeding 500,000/µL. The patient's underlying conditions included pneumonia and haemorrhagic stroke, which significantly increased the risk of morbidity and mortality in comparison to indivi- duals with normal platelet levels. These disorders have emerged as a distinct factor that can predict mortality within 30 days of receiving medical attention. The patient received initial treatment in the Intensive Care Unit (ICU) for a duration of two weeks. Subsequently, the patient was transferred to a standard hospital room. Unfortunately, the patient's condition deteriorated, and he passed away on the twentieth day of receiving medical care.
CONCLUSION
This case report has illustrated the clinical manifestation of a haemorrhagic stroke accompanied by reactive thrombocytosis. Reactive thrombocytosis is a result of specific underlying diseases, with acute infections being the primary cause of this type of thrombocytosis. Pneumonia is the primary infection associated with reactive thrombocytosis. Reactive thrombocytosis can be caused by acute bleeding, such as haemorrhagic stroke. Conversely, thrombocytosis can lead to the development of haemorrhagic stroke. Moreover, patients experiencing hemorrhagic stroke who subsequently develop thrombo- cytosis have been reported to exhibit an elevated predicted mortality risk.
LIST OF ABBREVIATIONS
| MPN | = Myelopro-liferative Neoplasms |
| JAK2 | = Janus Kinase 2 |
| WBC | = White Blood Cell |
CONSENT FOR PUBLICATION
Written informed consent has been taken from the patient’s family for this publication.


