Exacerbation of Mirror Movement Disorder: A Complex Case of Childhood-onset Mirror Movements Aggravated by Essential Tremors, and Parkinson's Disease
Abstract
Background
Mirror movement (MM), also known as bimanual synkinesis, is characterized by simultaneous involuntary movements of homologous muscles accompanying voluntary movements of contralateral body regions. MM can be observed in various neurological conditions, including cerebral palsy, corticobasal syndrome, Parkinson's disease, certain types of symptomatic epilepsies, Creutzfeldt-Jakob's disease, Huntington's disease, and others.
Case Description
A 52-year-old female with a history of epileptic seizures, essential tremors and mild MM was presented to a neurology clinic for seizure management. She had a long-standing history of seizures since childhood. Initial neurological examination revealed mild MM disorder. During the follow up visits, patient’s mirror movements were exacerbated months before she was diagnosed with Parkinson’s disease. Multiple antiepileptic drug regiments along with Carbidopa-levodopa were used and dose adjustments were done during follow up visits to help stabilize the patient’s symptoms.
Conclusion
In conclusion, this case highlights the progressive nature of mirror movement disorder (MMD) and its association with other neurological conditions, such as epilepsy, essential tremors, and Parkinson's disease. The primary aim of this study was to showcase that individuals with early childhood onset of MMD may experience a deterioration of their condition as they acquire additional neurological disorders, such as essential tremors or Parkinson's disease. Moreover, this study seeks to elucidate how the exacerbation of mirror movement symptoms in such cases can serve as an early indicator of the onset of Parkinson's disease. Medication adjustments played a crucial role in managing the patient's symptoms, emphasizing the importance of individualized treatment plans and close monitoring.
1. INTRODUCTION
Mirror movement (MM), also referred to bimanual synkinesis, is simultaneous, involuntary movement of homologous muscles that accompany voluntary move-ments of contralateral body regions. Mirror movements have been observed in various neurological conditions, including cerebral palsy, corticobasal syndrome (CBS), Creutzfeldt-Jakob's disease (CJD), and Huntington's disease, cervicomedullary junction anomaly (specifically Klippel-Feil syndrome), cerebrovascular disease, Parkin-son's disease, certain types of symptomatic epilepsies, Friedreich's ataxia, phenylketonuria, amyotrophic lateral sclerosis, Kallmann's syndrome, high cervical spinal cord malformation, alien hand syndrome (associated with vascular lesions), and certain psychiatric disorders such as obsessive-compulsive disorder and schizophrenia [1, 2].
MM and epilepsy, while distinct neurological conditions, may share overlapping pathophysiological mechanisms. Both disorders involve abnormal neural activity, potentially stemming from genetic predisposi-tions, structural abnormalities of the brain, or even the influence of antiepileptic medications. In individuals with epilepsy, the communication between the brain's hemis-pheres is altered, leading to an inability to suppress MMs. For example, if a person with epilepsy tries to move their right hand, the corresponding movement may also occur in their left hand. This simultaneous mirroring of movements can range from subtle to more pronounced, depending on the severity of the condition. It is important to note that MMs in epilepsy can coexist with other symptoms and can vary based on the type and location of the epileptic activity within the brain.
Familial cases have been reported with either autosomal-dominant or autosomal-recessive inheritance patterns. The prevalence of congenital movement disorder is less than one in one million. The involved genes are DCC (deleted in colorectal cancer), RAD5 (a DNA repair protein) and possibly DNAL4 (Dynein Axonemal Light Chain 4) [3]. The presence of MM can disrupt the coordinated movement of both hands, making tasks that require independent hand actions more challenging. They can hinder activities like tying shoelaces, cutting vegetables, typing, or buttoning shirts [1].
Patients can suffer from occasional pain in the upper limbs during sustained manual activities such as writing. The mirror movements usually persist throughout life, without deterioration or improvement and are not associated with any subsequent onset of additional neurological manifestations.
Mirror movements can occur in early childhood but usually resolve by age 10; however, persistent mirror movements can be seen in adulthood with congenital conditions such as Klippel-Feil syndrome and Kallman syndrome [4]. Klippel-Feil syndrome occurs as a result of failure of cervical segmentation, leading to fused cervical vertebrae. Mirror movements have been shown to be correlated to the severity of cervical fusion [5]. Kallmann syndrome is another congenital abnormality caused by failure of neural tracts to form, leading to anosmia and decreased gonadotropin releasing hormone (GnRH). In addition to symptoms of hypogonadotropic hypogonadism, about 40% of people with Kallmann syndrome also present with mirror movement disorders [6]. It has been shown that patients with Kallmann syndrome and mirror movements have hypertrophied corticospinal tracts due to abnormal development [7].
Mirror movements also occur in 29-95.7% of people with Parkinson’s disease but are more common in patients who are less severely affected by Parkinson’s disease [8]. Mirroring movements have been found to be more common in patients with asymmetric Parkinson’s disease, occurring more commonly in the less-affected limb. MM severity has been correlated to the degree of asymmetry of motor deficits, usually affecting the upper limbs [9]. A study conducted by Chatterjee et al. suggested 95.7% of patients with Parkinson’s disease had MM, and symptoms of MM were found to be more severe in patients with decreased severity of Parkinson’s disease [10]. Addition-ally, Ottaviani et al. demonstrated similar findings in which there was a significant inverse correlation between the severity of MM symptoms and the progression of Parkinson’s disease [11]. MMs are an early sign of Parkinson’s disease and tend to diminish in severity as the disease progresses.
Amyotrophic lateral sclerosis (ALS) is another progressive neurodegenerative disorder characterized by progressive loss of the upper and lower motor neurons (LMNs) at the spinal or bulbar level [12]. Mirror movements occur in approximately 25-39% of people with ALS and usually occur early in the disease [8]. Compared to Parkinson’s disease, mirror movements are correlated with increased symptomology in ALS [13].
Mirror movements can also be present in people with essential tremors, a movement disorder that commonly affects the upper limbs [14]. Several studies have shown the prevalence of mirror movements in patients with essential tremors to be between 32.7 and 77.7% [8]. Mirror movements in essential tremor cases are most common and severe, with rest tremor and more prevalent in the hands [15].
While the Woods Teuber scale continues to be the accepted universal standard for evaluating MM across a broad spectrum of illnesses, the Unified Parkinson's Disease Rating Scale and United Huntington's Disease Rating Scale may be helpful for evaluating MM within PD and HD, respectively. The Woods Teuber classification may benefit from the addition of transcranial magnetic stimulation (TMS) and electric muscular stimulation (EMS) studies, which can aid to pinpoint the location and intensity of mirror muscle contractions. TMS which uses electromagnetic induction to generate electric currents in specific regions of the brain, could potentially contribute to mapping out the neural pathways involved in mirror muscle contractions, helping to pinpoint the specific brain areas contributing to these movements [16]. EMS, which uses electrical currents to stimulate muscle contractions, could be utilized to mimic mirror muscle contractions in a controlled manner. Through adjusting the intensity of EMS, one could potentially investigate how varying levels of stimulation impact the patterns of muscle activation and mirror movements. There are several different muscle groups that have been used to measure MM; however, since finger tapping is a common test for MM, contraction of the hand muscles, such as the first dorsal interosseous muscle (FDI), is valuable for research. A more homogeneous study methodology would improve the discussion of MM and, ideally, result in a deeper comprehension of this phenomenon [1].
Not many studies dive into possible treatments for this rare condition and few case reports have specifically addressed the management of MMs. Allegra et al. reported on the case of the successful treatment of a patient with a congenital mirror movement disorder (CMM) using the botulinum toxin (BoNT). This study demonstrated that BoNT, which targets specific muscles and allows a reduction in MMs in noninjected muscles, may be effective for the treatment of MMs. This approach was confirmed by the polygraph EMG recording, which revealed a decrease in mirror EMG activity in the right flexor digitorum superficialis muscle that had not received a BoNT injection. The underlying mechanism for this remains unknown. It is anticipated that BoNT can influence sensory afferents, modifying the signal from muscle spindles, affecting cortical networks in the process, and rectifying the physiological lateralization of the motor program, in contrast to the abnormal ipsilateral pathways identified in CMMs [17].
The principal objective of this case study is to illustrate that individuals who experience MMD with a childhood onset can witness a deterioration in their condition as they develop other neurological disorders, including essential tremors or Parkinson's disease. Furthermore, this case report aims to elucidate the significance of this exacerbation of mirror movement symptoms in such cases as an early indication of the emergence of Parkinson's disease. The exacerbation of MMD observed in a patient with an onset in childhood, coupled with the subsequent development of accompanying neurological disorders, emphasizes the necessity for a more profound comprehension of the fundamental pathophysiological mechanisms at play.
2. CASE DESCRIPTION
A 52-year-old right hand dominant female presented to outpatient Neurology clinic for evaluation of her epileptic seizures and essential tremors. The patient had a history of seizures since childhood, around the age of 6 or 7, often characterized by sudden loss of consciousness, urine incontinence, and tongue biting. Postictal headaches and muscle aches were noted. Seizures occurred sporadically, with the most recent one taking place a few months before the initial evaluation. No specific triggers or history of head trauma were identified. She was taking 1000 mg of primidone daily, which exceeded the maximum recommended dose.
Initial neurological exam findings were as follows: Mental Status: alert and oriented to person, place and event but not to date. Recall and attention-concentration were intact. Cranial nerves: II-XII were normal bilaterally. Motor Strength: Moved all extremities with motor function score 5 out of 5 throughout. Reflexes: normal/symmetric; Sensory: hypesthesia to pin prick throughout. Coordi-nation: finger to nose and heel to shin were symmetrically intact. Gait: difficulty tandem. Initial physical exam also revealed mild mirror movement disorder. To confirm MMs, the Woods and Teuber scale (W&T) was utilized. Patient was asked to perform repetitive tapping of the index finger on the thumb, alternating supination and pronation of the hand, repetitive alternate touching of each fingertip and rapid ankle flexion–extension while we measured mirror movements observed in resting left side extremities [18]. Patient reported having mirror movements in upper extremities since childhood.
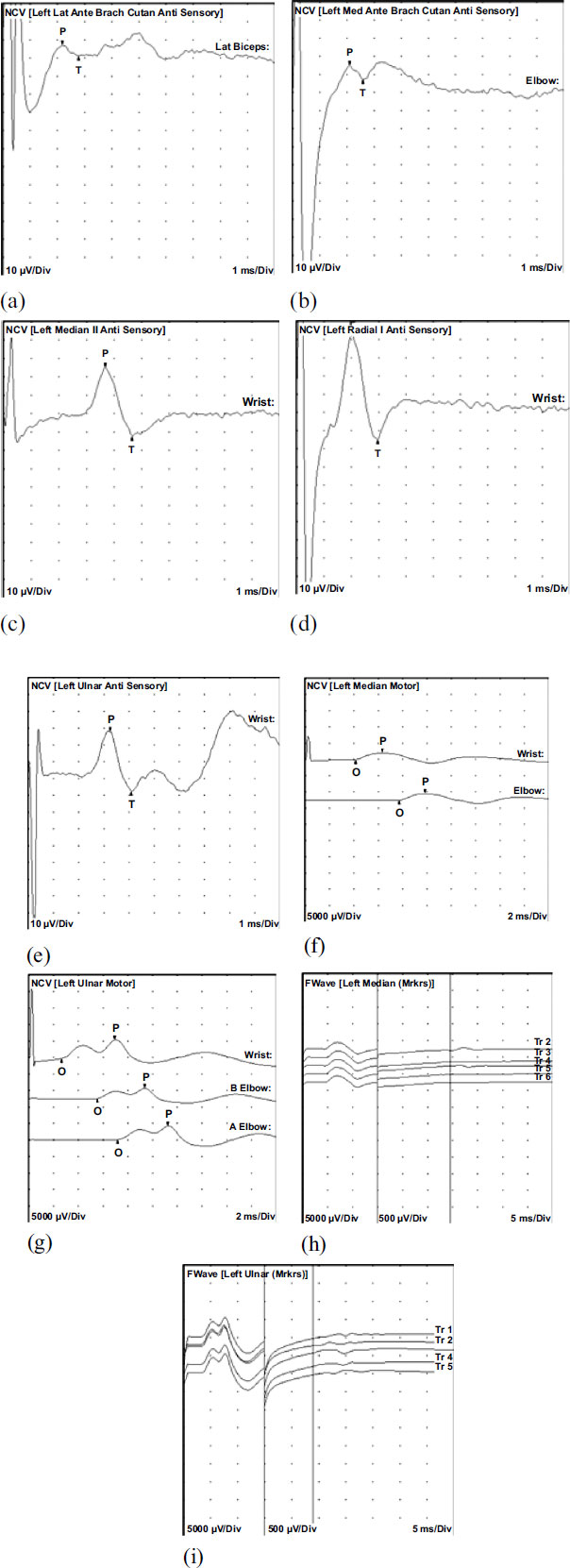
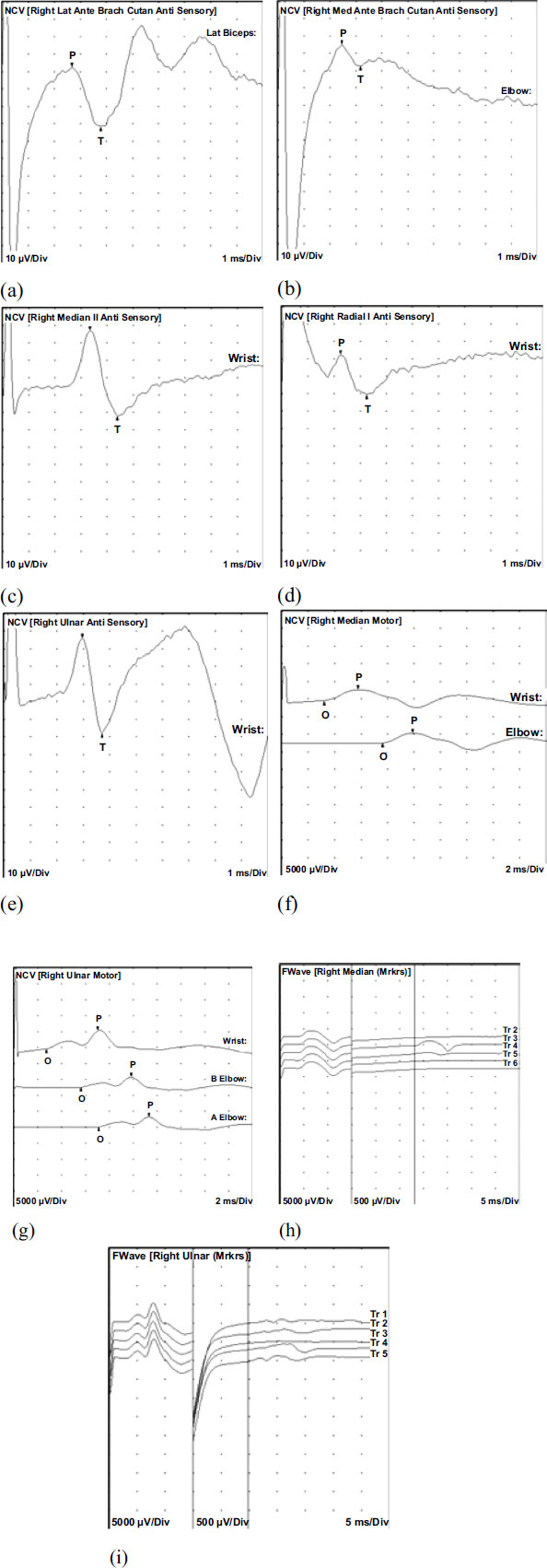
Further evaluation of speech patterns, facial expressions, eye movements, posture, and body move-ments revealed psychomotor retardation. The initial evaluation included an EEG, which revealed generalized epileptiform discharges during one episode. MRI of the brain showed no abnormalities. A nerve conduction study (Figs. 1 and 2) and EMG (Table 1) indicated mild sensori-motor polyneuropathy of the upper extremities, suggesting potential underlying etiologies such as vasculitic, diabetic, metabolic, nutritional, toxic, or inflammatory factors (Figs. 1 and 2). Subsequent EEGs did not show any significant changes. To manage the seizures, the patient's primidone dosage was reduced to 750 mg daily, and she was started on Lacosamide 50 mg twice daily. However, the patient reported increased headaches with Lacosamide, leading to its discontinuation. Levetiracetam 500 mg twice daily was initiated instead. Additionally, the patient experienced fatigue and memory impairment, which were addressed as potential iatrogenic effects. During follow-up visits, she continued to report tiredness despite the reduction in primidone dosage and the initiation of new medications. Symptoms of imbalance, falls, muscle cramps and tingling/numbness in the hands were also noted. During subsequent visits, her Mirror movement was more prominent and W&T scaling illustrated a stronger but briefer, repetitive movements of resting left hand while right hand was moving. No mirror movement was observed in lower extremities. In subsequent follow-up visits patient presented with bradykinesia, rigidity of the upper extremities and resting tremors. Patient was eventually diagnosed with Parkinson’s disease and was started on Carbidopa-levodopa 25-100 mg, 1 tablet by mouth three times a day.
A medication dose adjustment was made at subsequent visits to improve her symptom control. Patient stayed on the same medication regimen for a year during her follow-up visits. Her antiepileptic drugs were Primidone 250mg, one tablet three times a day, Topiramate 100mg one tablet twice daily, and she continued the same dosage of Carbidopa-levodopa since her Parkinson’s’ disease symptoms were managed. Her mirror movements remained stable during the follow-up visits for the next 12 months.
The left medial antebrachial cutaneous sensory showed normal distal peak latency (2.1 ms), normal amplitude (7.5 µV), and decreased conduction velocity (Elbow-Forearm, 48, m/s). The feft median motor nerve showed prolonged distal onset latency (4.1 ms), reduced amplitude (2.2 mV), and normal conduction velocity (Elbow-Wrist, 53 m/s). The Left Median II sensory nerve showed normal distal peak latency (3.7 ms), normal amplitude (37.3 µV), and decreased conduction velocity (Wrist-2nd Digit, 38 m/s). The Left ulnar motor nerves showed normal distal onset latency (2.7 ms), reduced amplitude (5.7 mV), normal conduction velocity (B Elbow-Wrist, 54 m/s), and normal conduction velocity (A Elbow-B Elbow, 65 m/s). All remaining nerves were within normal limits. All F Wave latencies were within normal limits.
Evaluation of the Right lateral antebrachial cutaneous sensory nerve showed normal distal peak latency (2.7 ms), normal amplitude (34.3 µV), and decreased conduction velocity (Lat Biceps-Lat Forearm, 44 m/s). Right medial antebrachial cutaneous sensory nerves showed normal distal peak latency (2.5 ms), normal amplitude (12.0 µV), and decreased conduction velocity (Elbow-Forearm, 40 m/s). The Right median motor nerve showed normal distal onset latency (3.2 ms), reduced amplitude (3.2 mV), and decreased conduction velocity (Elbow-Wrist, 47 m/s). Right ulnar motor nerves showed normal distal onset latency (2.7 ms), reduced amplitude (5.8 mV), normal conduction velocity (B Elbow-Wrist, 55 m/s), and normal conduction velocity (A Elbow-B Elbow, 80 m/s). All remaining nerves were within normal limits. All F Wave latencies were within normal limits.
| Side | Muscle | Nerve | Root | Ins Act | Fibs | Psw | Other | Amp | Dur | Poly | Recrt | Int Pat |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Right | 1stDorInt | - | - | Nml | Nml | Nml | Nml | Nml | Nml | Nml | Nml | Nml |
| Right | ExtDigCom | - | - | Nml | Nml | Nml | Nml | Nml | Nml | Nml | Nml | Nml |
| Right | BrachioRad | Radial | C5-6 | Nml | Nml | Nml | Nml | Nml | Nml | 1+ | Nml | Nml |
| Right | Triceps | Radial | C6-7-8 | Nml | Nml | Nml | Nml | Nml | Nml | 1+ | Nml | Nml |
| Right | Deltoid | Axillary | C5-6 | Nml | Nml | Nml | Nml | Nml | Nml | Nml | Nml | Nml |
| Left | 1stDorInt | - | - | Nml | Nml | Nml | Nml | Nml | Nml | 1+ | Nml | Nml |
| Left | ExtDigCom | - | - | Nml | Nml | Nml | Nml | Nml | Nml | Nml | Nml | Nml |
| Left | BrachioRad | Radial | C5-6 | Nml | Nml | Nml | Nml | Nml | Nml | Nml | Nml | Nml |
| Left | Triceps | Radial | C6-7-8 | Nml | Nml | Nml | Nml | Nml | Nml | 1+ | Nml | Nml |
| Left | Deltoid | Axillary | C5-6 | Nml | Nml | Nml | Nml | Nml | Nml | 1+ | Nml | Nml |
3. DISCUSSION
The purpose of this case study is to illustrate that within the cohort of patients presenting with an early childhood onset of MMD, their condition can deteriorate upon the acquisition of other neurological disorders, such as epilepsy, essential tremors or Parkinson's disease. Additionally, this case report endeavors to elucidate how the progression of mirror movement impairment in such instances can serve as an early indicative marker for the emergence of Parkinson’s disease.
The severity of mirror movement was quantified using established clinical scales, illustrating a significant escalation over time. Given the patient's long-standing mirror movement disorder since childhood, an exploration of the potential pathophysiological mechanisms within the brain becomes pertinent as the patient develops additional neurological disorders, which contribute to the exacerbation of mirror movements.
The pathophysiology of congenital mirror movement disorder (CMM) is theorized to be due to the interaction of uncrossed fast-conducting corticomotoneuronal projections with unintentional motor activity from the primary motor cortex (M1) on the opposite side of the affected mirror motor cortex [19]. The exact mechanism by which CMM can lead to parkinsonism is not fully understood but several mechanisms have been theorized. One study postulated that the normal balance and coordination of movements are disrupted by the aberrant bilateral motor activity and cross-communication between the motor cortices, leading to symptoms such as bradykinesia, rigidity and tremors [20].
Mirror movement disorder has been identified as a clinical feature in patients with early, asymmetric Parkinson's disease (PD). A study involving 27 patients with early PD found that MMD was present in 24 of the patients. There was a significant correlation between the degree of asymmetry in motor deficits and the occurrence of MMD on the less affected side. The presence of MMD may be a useful clinical finding for the early detection of PD, particularly in cases of mild asymmetric parkinsonism. Additionally, leg rigidity asymmetry was found to be a significant predictor of contralateral foot mirroring. Further research is needed to better understand the association between MMD and PD, as well as the underlying mechanisms involved [9]. In this case, the patient did not have any MM in her lower extremities. However, her MM was exacerbated a few months before she was diagnosed with PD.
MMD in PD is likely caused by a combination of factors. As reported by Liu et al., disrupted interhemispheric inhibition, increased motor cortex excitability in the ipsilateral hemisphere, and alterations in cortical and subcortical circuits contribute to the development of MMD [8]. Specifically, decreased interhemispheric inhibition leads to excessive motor output from the ipsilateral hemisphere through crossed pathways, resulting in mirroring movements. Dysfunction in the striatal-cortical circuit is also implicated in motor deficits, including MMD, in PD. These complex mechanisms contribute to the occurrence of MMD in PD patients [8].
The correlation of MMD with essential tremor (ET) is not yet well understood. However, studies have reported the presence of MMD in a significant percentage of ET cases. MMD in ET patients appears to be more common and severe in cases with resting tremor. It is worth noting that some evidence suggests a potential link between ET and PD, as some ET patients may have an increased risk of developing PD. However, the specific pathophysiological mechanisms responsible for MMD in ET and their relation to the development of PD require further investigation. Longitudinal cohort studies are needed to better understand the connection between MMD, ET, and the progression to PD [8]. According to Cox et al., ET causes disruptions in the cerebral networks responsible for unilateral movement within one hemisphere, which may be a probable mechanism for MMD [1].
Mirror movements can also be observed in individuals with epilepsy. In a study of a case involving symptomatic epilepsy and MM caused by unilateral schizencephaly, it was suggested that the damaged right cerebral hemisphere may have reorganized the unaffected brain by establishing a transcallosal inhibitory channel. This reorganization could have disinhibited the pyramidal tract in the left cerebral hemisphere, leading to mirror movements observed in the patient's left hand and leg [21].
Due to the prolonged usage of antiepileptic drugs such as primidone in this case, it becomes imperative to emphasize the distinct criteria for distinguishing among the diagnoses of Parkinson's disease, essential tremor, and tardive dyskinesia. For instance, in the case of Parkinson's disease, patients typically exhibit bradykinesia, resting tremor, and rigidity, which usually show a positive response to dopaminergic therapy. In contrast, Essential Tremor is characterized by an isolated action tremor, often affecting the hands, with an absence of other parkinsonian features. Furthermore, Tardive Dyskinesia, which is often linked to extended use of specific medications like primidone, is marked by involuntary and repetitive movements, often seen in the facial region. It's important to note that tardive dyskinesia may not exhibit a favorable response to dopaminergic therapy. Dopamine transporter single-photon emission computed tomography scan (DaTscan) is a diagnostic imaging technique employed in diagnosing movement disorders such as Parkinson’s disease. DaTscan utilizes a radiotracer that attaches to dopamine transporters. In Parkinson's disease, the loss of dopamine-producing neurons results in a notable decrease in dopamine transporters. Therefore, DaTscan imaging displays a pronounced reduction in the uptake of the radioactive tracer in the striatum [22]. However, in other movement disorders like Essential Tremor or Tardive Dyskinesia, DaTscan would reveal intact dopamine transporters [23]. Although not utilized in this specific case study, DaTscan could have contributed to discerning the specific movement disorder type.
MMD is frequently regarded as a symptom or presentation of an underlying neurological disorder rather than as a distinct illness. As a result, there is a strong correlation between the prognosis and the primary movement disorder. While some people's MMD may remain stable or improve gradually over time, others may experience it deteriorating as the underlying condition worsens. In some cases, medications can have a significant impact on mirror movement disorders. People on dopaminergic therapy for Parkinson’s disease with improvement of motor symptoms may experience more severe mirroring movements [8]. Although the effect of dopaminergic therapy on mirror movements is not well understood, it is hypothesized the improvement in bradykinesia and rigidity in the less affected limb, unmasks mirror movements [24].
In our case ongoing medication adjustments were made based on the patient's response and tolerability. In addition to addressing the seizures, essential tremors and Parkinson’s disease, management of the patient's other symptoms was essential. The patient reported fatigue, memory impairment, muscle aches, and sensory abnormalities. These symptoms were evaluated and managed through medication adjustments, physical therapy, and referral to appropriate specialists. Vitamin supplementation was also considered to address potential nutritional deficiencies contributing to the symptoms. Close monitoring of medication response, side effects, and ongoing symptom assessment was crucial for guiding treatment decisions.
CONCLUSION
This case report highlights the escalating severity of mirror movement in an individual with a history of childhood epilepsy, subsequently developing additional neurological disorders such as essential tremors and Parkinson's disease later in life. The investigation sheds light on the intricate interplay between these conditions and underscores the importance of comprehensive management strategies to address the cumulative impact on motor function.
The patient's quality of life and her symptoms control were greatly enhanced by mitigative measures such as assessment monitoring, medications, and symptom management despite the emergence of new symptoms and comorbidities. Further research is warranted to elucidate the underlying mechanisms and develop targeted interventions that effectively alleviate mirror movement symptoms in individuals with complex comorbidities.
AUTHORS' CONTRIBUTIONS
All authors contributed to all sections. All authors have read and agreed to the published version of the manuscript.
LIST OF ABBREVIATIONS
| MM | = Mirror movement |
| MMD | = Mirror movement disorder |
| CBS | = Corticobasal syndrome |
| CJD | = Creutzfeldt-Jakob's disease |
| ALS | = Amyotrophic lateral sclerosis |
| LMNs | = Lower motor neurons |
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
Not applicable.
HUMAN AND ANIMAL RIGHTS
Not applicable.
CONSENT FOR PUBLICATION
Informed consent was obtained from subject involved in the study.
STANDARDS OF REPORTING
CARE guidelines were followed.
AVAILABILITY OF DATA AND MATERIALS
The data and supportive information is available within the article.
FUNDING
None.
CONFLICT OF INTEREST
The authors declare no conflict of interest financial or otherwise.
ACKNOWLEDGEMENTS
Declared none.


