All published articles of this journal are available on ScienceDirect.
Effect of Sodium Valproate Treatment on the Cardiac Index in New Cases with Status Epilepticus
Abstract
Background:
Sodium valproate is an antiepileptic drug primarily used to treat status epilepticus [SE]; however, its effect on cardiac function is unclear. This study aimed to examine the effect of 6 months of sodium valproate treatment on the cardiac index in new cases with status epilepticus.
Methods:
In this cross-sectional study, 30 cases with status epilepticus [18 boys and 12 girls] who were admitted to the Pediatric Intensive Care Unit of Hazrat-e Rasool Hospital were enrolled. Information on basic demographic and clinical data of all children, such as age, weight, gender, blood pressures, and underlying diseases, was recorded. Echocardiography and electrocardiogram [ECG] were performed for all cases before and after the treatment.
Results:
There were no abnormalities in ECG parameters [including PR, QRS, and QT intervals] after 6 months of treatment with sodium valproate. No significant differences were found in echocardiographic parameters, including blood pressure, pulmonary artery pressure [PAP], right ventricular [RV] size, diastolic dysfunction,], Tie index, end-diastolic volume [EDV], ejection fraction [EF], and TAPSE before and after study [p>0.05].
Conclusion:
Administration of sodium valproate over 6 months is not associated with a serious adverse effect on heart function in children with status epilepticus.
1. INTRODUCTION
Epilepsy is a common chronic neurological disorder and a leading cause of mortality in Children. It is estimated that 0.8% of children under the age of 15 have active epilepsy and more than 80% of them live in developing countries [1]. Status epilepticus (SE) is a condition in which a seizure lasts equal to or more than 30 minutes [2]. Currently, it is defined as a condition caused by the defects in factors responsible for seizure termination or from the initiation of mechanisms that lead to abnormally prolonged seizures [3]. In the United States, SE represents 0.07% of hospital admissions [4]. While short-term seizures generally do not damage the brain, SE is associated with permanent brain damage, resulting in severe negative consequences and death in children [5]. Therefore, active and effective management of this life-threatening disease is critical for saving the lives of children.
Till now, several antiepileptic drugs have emerged, bringing new hope for the treatment of epilepsy. Sodium valproate is a widely used antiepileptic drug that treats epilepsy by preventing seizures through a broad spectrum of activity and mechanism of action [6]. It has been proposed that its action is probably mediated through the function of brain gamma-aminobutyric acid (GABA) [7]. Valproic acid exhibits its pharmacologic effects in a couple of ways, such as by acting on GABA [γ aminobutyric acid] levels in the CNS, blocking voltage-gated ion channels, and inhibiting histone deacetylase. Impaired GABAergic inhibitory activity is an established pathophysiology of seizure initiation and propagation, given that controlling this pathway is a potential target for antiepileptic drugs.
A growing number of studies have recommended the use of sodium valproate for treatment and control of SE due to its higher tolerability and lower hemodynamic instability than other antiepileptic drugs [8, 9]. Although many studies can be considered a safe and effective option in the management of SE, it may be associated with several side effects. Like other anticonvulsant drugs, sodium valproate has several side effects such as excessive weight gain, possibly by stimulating appetite, drowsiness causing the patient to be sleepy, hair loss (a reversible side effect that usually can be observed 3 months after starting dose or later. In some cases, it takes 1 year or 2 years before hair loss), tremor approximately 25% of the patients develop a tremor within 3–12 months of initiating therapy and often observed with action or postural in nature, although a resting tremor may occur. The tremor can affect a patient's head, mouth, tongue, and limbs, and reversible thrombocytopenia occurred in 12–18% of subjects receiving treatment. Advanced age, female gender, and high doses were found to be risk factors for the development of thrombocytopenia, increased level of liver enzymes (gamma-glutamyl transferase, alanine aminotransferase, and aspartate amino transferase), hepatotoxicity and acute liver failure, encephalopathy due to induced hyperammonemia, coagulation disorders in the field of liver failure or platelet dysfunctions, pancreatitis and bone marrow suppression [10-13]. Sarnacchiaro et al. reported that Sodium Valproate at the dose of 400 mg induced a significant decrease of serum Prolactin in healthy subjects (p < 0.05), whereas no effect was noted in both tumoral and non-tumoral hyperprolactinemia [14].
Sodium valproate interacts with drugs such as phenobarbital, phenytoin, carbamazepine, lamotrigine, rifampin, ethosuximide, and primidone [15]. Recent studies have supposed that sodium valproate may result in cardiac failure in children [16]; however, its effect on cardiac function is still controversial. Therefore, researching the effect of sodium valproate on heart function and structure in children is valuable for the prevention of heart problems in these cases. This study aimed to investigate the effect of 6-month treatment of sodium valproate on cardiac indices in new cases with status epilepticus.
2. METHODS
In this cross-sectional study, 30 new cases with SE who were admitted to Pediatric Intensive Care Unit (PICU) at Hazrat e Rasool Hospital (Tehran, Iran) during 2019-2020 were entered into the study. The study was approved by the Ethics Committee of the Iran University of Medical Sciences. All parents signed a consent letter before the study. At the beginning of the study, a checklist was provided in which information on basic demographic and clinical data of all children, such as age, weight, gender, blood pressures (SBP and DBP), and underlying diseases were recorded. Only new cases of SE with normal biochemical and hematological test results were entered into the study. Patients with a history of congenital disorders (e.g., heart abnormalities), diabetes, chronic respiratory diseases, cancer, and a history of using anti-SE drugs were excluded from the study. In total, 30 children (60% boys and 40% girls) with a mean age of 6.24 ± 2.46 years and a mean weight of 26.12 ± 2.46 kg were entered into the study. After the patient’s selection, echocardiography was performed for all selected cases and parameters such as pulmonary artery pressure (PAP), right ventricular (RV) size, LV end-diastolic volume (EDV), diastolic function, Tie index, ejection fraction (EF), and TAPSE were recorded. Then, patients were treated with sodium valproate for 6 months and monitored by a physician during this period. At the end of the study, patients were referred to the hospital and the second echocardiography was performed for all cases. Additionally, electrocardiogram (ECG) parameters (e.g., QRS, QT, and PR) were recorded for each patient at the end of the study. Eventually, the mean of echocardiography and ECG parameters before and after the study were compared.
2.1. Statistical Analysis
All data were analyzed by SPSS software (IBM, version 19). Quantitative data were presented as Mean ± SD. The Chi-Square test was used to compare the percentage or frequencies of qualitative data. Comparison of the mean of parametric data between before and after the study was analyzed using the paired t-test, while the Wilcoxon test was applied for the analysis of non-parametric data. A p< 0.05 was considered statistically significant.
3. RESULTS
In total, 30 children were enrolled in the study. There was no significant difference in the mean of SBP and DBP between before and after the study. The mean of SBP before and after the study was 102.14 ± 9.94 mmHg and 105.28 ± 10.54 mmHg, respectively (p=0.09). The mean of DBP before and after the study was 62.14 ± 7.77 mmHg and 61.07 ± 6.84 mmHg, respectively (p=0.53).
The mean of raw quantitative echocardiographic parameters is presented in Table 1. There was no significant difference in the mean of each quantitative echocardiographic parameter before and after the study. No significant difference was found between the frequency of PAP (p=0.31) and RV size (p=0.56) before and after the study. All patients (100%) had normal RV and global functions before and after the study. There was no significant difference in diastolic function status before and after the study (p=0.96). The frequency of patients with diastolic dysfunction was 4.5% and 9.1% before and after the study, respectively (Fig. 1).
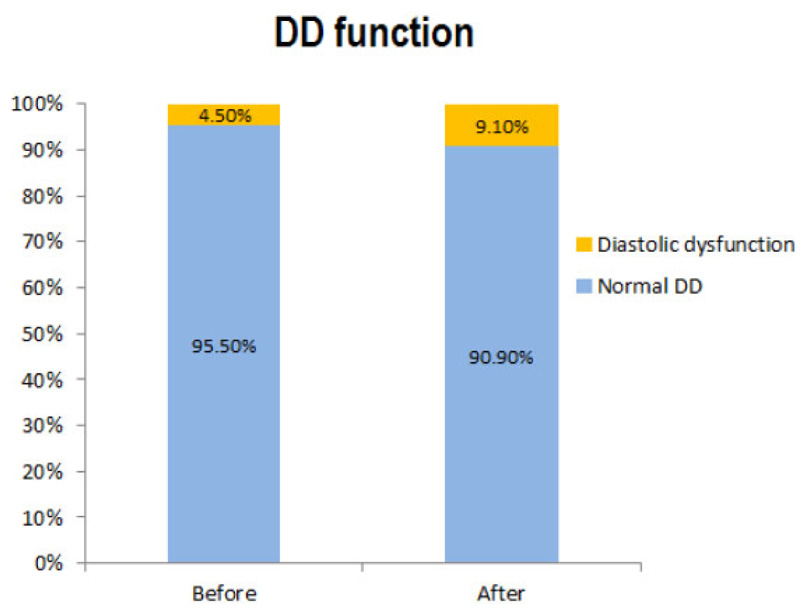
| Variables | Before | After | p-value |
|---|---|---|---|
| LADmax | 4.15 ± 1.09 | 3.9 ± 1.1 | 0.38 |
| LA area 1 | 7.35 ± 2.45 | 7.66 ± 3.01 | 0.095 |
| LA area 2 | 8.44 ± 2.72 | 8.37 ± 2.13 | 0.77 |
| E | 105.51 ± 13.83 | 104.12 ± 10.57 | 0.88 |
| A | 66.58 ± 17.36 | 66.4 ± 10.45 | 0.57 |
| E/A | 1.68 ± 0.48 | 1.6 ± 0.31 | 0.1 |
| DT | 97.66 ± 22.04 | 100.83 ± 20.46 | 0.61 |
| Septal E | 11.87 ± 2.12 | 11.58 ± 2.76 | 0.69 |
| Septal A | 6.87 ± 1.96 | 6.26 ± 1.92 | 0.3 |
| Septal S | 6.98 ± 1.3 | 6.9 ± 1.12 | 0.81 |
| Lateral E | 14.66 ± 2.83 | 15.07 ± 2.99 | 0.64 |
| Lateral A | 7.84 ± 2.62 | 7.75 ± 2.62 | 0.91 |
| Lateral S | 7.7 ± 1.62 | 7.66 ± 1.3 | 0.92 |
| Tie index | 0.39 ± 0.16 | 0.34 ± 0.054 | 0.37 |
| LA/AO | 1.02 ± 0.09 | 1.0 ± 0.07 | 0.68 |
| RVIDd | 1.30 ± 2.17 | 1.72 ± 2.74 | 0.3 |
| IVSd | 1.29 ± 1.75 | 1.29 ± 1.8 | 0.99 |
| LVIDd | 6.32 ± 8.32 | 6.33 ± 8.2 | 0.99 |
| LVPWd | 1.18 ± 1.59 | 1.19 ± 1.64 | 0.97 |
| LVIDs | 3.39 ± 4.26 | 3.48 ± 4.28 | 0.94 |
| LVPWs | 1.49 ± 1.93 | 1.55 ± 1.98 | 0.91 |
| ESV | 40.08 ± 19.36 | 40.64 ± 18.06 | 0.92 |
| EDV | 15.03 ± 7.91 | 15.54 ± 7.59 | 0.82 |
| FS | 35.2 ± 10.89 | 32.15 ± 6.5 | 0.26 |
| EF | 63.2 ± 5.97 | 61.0 ± 13.24 | 0.33 |
| Lvmass | 60.09 ± 25.68 | 54.78 ± 19.11 | 0.46 |
| RV1 | 3.49 ± 1.14 | 3.72 ± 1.06 | 0.5 |
| RV2 | 3.11 ± 4.02 | 2.38 ± 0.4 | 0.4 |
| RV3 | 5.31 ± 1.62 | 5.27 ± 1.37 | 0.92 |
| TAPSE | 2.59 ± 0.41 | 2.54 ± 3.17 | 0.66 |
| TRPG | 25.28 ± 3.71 | 25.9 ± 3.17 | 0.57 |
| PIPG | 14.13 ± 2.33 | 12.95 ± 1.52 | 0.054 |
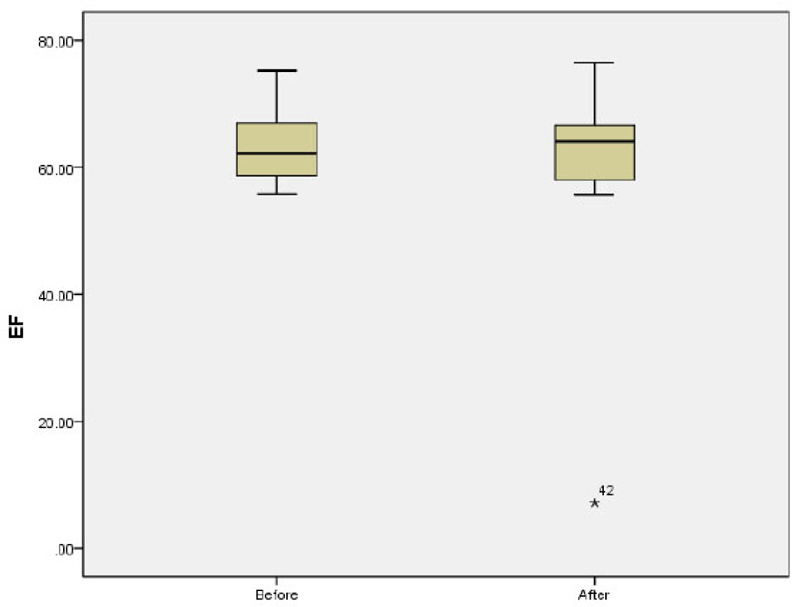
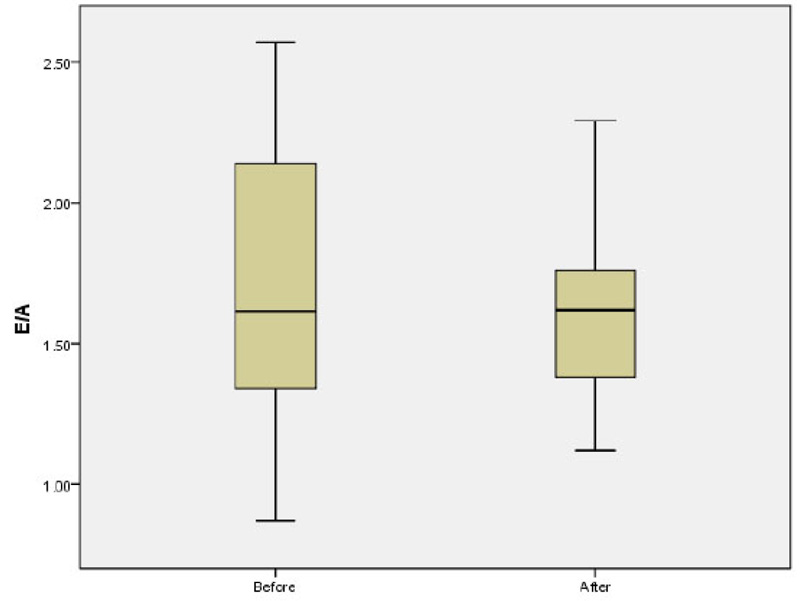
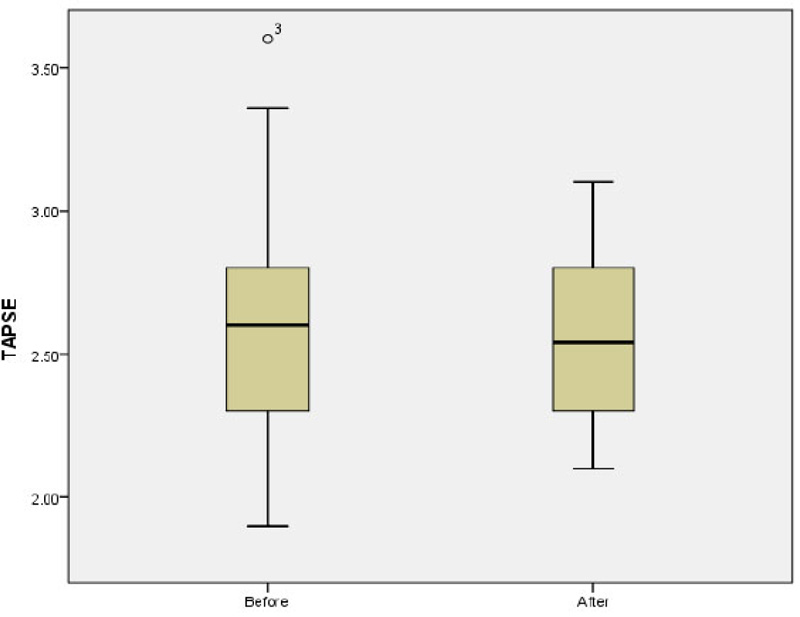
The mean of LAP before and after the study was 4.42 ± 0.35 and 4.44 ± 0.46, respectively (p=0.8). The mean E/A ratio before and after the study was 1.68 ± 0.48 and 1.6 ± 0.31, respectively p=0.1; (Fig. 2). No significant difference was observed in the mean of LAV before and after the study (13.05 ± 6.22 vs 13.23 ± 5.72; p=0.5). The mean of Tie index before and after the study was 0.39 ± 0.16 and 0.34 ± 0.054, respectively (p=0.37). Further analysis also showed non-significant difference in the mean of EDV (40.08 ± 19.36 vs 40.64 ± 18.06; p=0.92) and ESV (15.03 ± 7.91 vs 15.54 ± 7.59; p=0.82) before and after study. The mean of EF before and after the study was 63.2 ± 5.97 and 61.0 ± 13.24 before and after the study, respectively p=0.33; (Fig. 3). There was no significant difference in the mean of TAPSE before and after the study 2.59 ± 0.41 vs. 2.54 ± 0.29; p=0.66; (Fig. 4).
4. DISCUSSION
Sodium valproate is one of the most efficient antiepileptic drugs; however, its effect on cardiac function has remained controversial [17]. In the present study, we investigated the effect of 6 months of treatment with sodium valproate on cardiac indices in new cases with status epilepticus. We did not observe abnormalities in ECG parameters (including PR, QRS, and QT intervals) after 6 months of treatment with sodium valproate. Furthermore, a non-significant difference or change was observed in echocardiographic parameters, including blood pressure, pulmonary pressure, RV size, RV function, global function, EF, and Tie index before and after sodium valproate therapy. These data indicate that treatment with sodium valproate to control the status epilepticus for 6 months does not harm cardiac function and structure in children. Therefore, it can be considered an effective drug in the management of SE in children. Moreover, in our study, the initial evaluation revealed several cases with a slight increase in pulmonary pressure, size of the left atrium, and diastolic dysfunction. These abnormalities may be related to the hypoxic effects of long-term seizures. Although there was no significant difference in the frequency of these variables before and after drug therapy, it emphasizes the need for echocardiographic analysis of patients who experienced prolonged seizures.
There are few studies with contradictory results that considered the effect of sodium valproate on cardiac indices. More recently, Kamani et al. [18] investigated the effect of sodium valproate (5-30 mg/kg) on cardiac function in 60 children with status epilepticus compared to normal children. Their findings did not show a significant difference in echocardiographic parameters such as TAPSE, EF, fractional shortening (FS), and LV enlargement between the two groups, which is consistent with the results of our study. These results support the fact that sodium valproate is beneficial to alleviate status epilepticus without causing cardiac complications in children. In another study, Tian et al. [19] examined the effect of sodium valproate administration in rats with myocardial infarction (MI). Their results showed that sodium valproate had a protective effect on the heart and significantly decreased heart damage after MI. Previous research reported that intravenous injection of sodium valproate significantly improved status epilepticus with no significant cardiovascular compromises such as hypotension [9].
Nevertheless, limited studies reported the negative effects of sodium valproate on cardiac function. For example, Özdemir et al. [16] examined the side effects of sodium valproate [20 mg/kg and 2 mg/kg/h-12h] in 19 adult patients with status epilepticus following ischemic stroke. They demonstrated that sodium valproate led to systemic side effects such as hypotension, abnormal heart rate, and respiratory failure in these patients. However, they did not perform electrocardiography and echocardiography for patients. A case report study, Sivananthan and Mohiuddin [20], showed that discontinuation of valproate caused blood pressure stabilization in an 8-year-old boy with hypertensive urgency. They concluded that the administration of valproate may be a risk factor for hypertension in children. In an experimental study, Wu et al. [21] reported that sodium valproate causes various fetal cardiac abnormalities in mice through inhibition of histone deacetylase.
CONCLUSION
Our findings revealed that administration of sodium valproate over 6 months did not lead to heart problems in children with status epilepticus. Thus, it can be concluded that sodium valproate is a useful medication to control status epilepticus in children without serious adverse effects on heart function. However, a major limitation of this study was the small number of subjects. Therefore, another study with a larger sample size and in a period longer than 6mo is valuable to confirm these findings.
AUTHORS' CONTRIBUTIONS
RM, VM designed and managed the study. NF analyzed and interpreted the patients' data and performed follow up of the patients. NF was major contributor in writing the manuscript. All of the authors read and approved the final manuscript.
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
This study was approved by the Ethics Committee of the Iran University of Medical Sciences. The ethical number was IR.IUMS.FMD.REC.1399.178.
HUMAN AND ANIMAL RIGHTS
No animals were used for studies that are base of this research. All the humans used were in accordance with the ethical standards of the committee responsible for human experimentation (institutional and national), and with the Helsinki Declaration of 1975, as revised in 2013 (http://ethics.iit.edu/ecodes/node/3931).
CONSENT FOR PUBLICATION
The consent for publication of personal detailed data was obtained from parents.
STANDARDS OF REPORTING
STROBE guidelines and methodologies were followed in this study.
AVAILABILITY OF DATA AND MATERIALS
The data supporting the findings of the article is available in the dropbox at: https://www.dropbox.com/home/3. Sodium Valproate and Heart Complications.
FUNDING
None.
CONFLICT OF INTEREST
The authors declare no conflict of interest, financial or otherwise.
ACKNOWLEDGEMENTS
We would also like to appreciate Hazrat-e Rasool Hospital and Iran University of Medical Sciences, Tehran, Iran, for their technical supports throughout the period of study.


