All published articles of this journal are available on ScienceDirect.
Posterior Reversible Encephalopathy Syndrome Following Blood Transfusion: A Rare Case Report with Literature Review
Abstract
Background:
Posterior reversible encephalopathy syndrome (PRES) is clinicoradiological disease entity characterized by headache, seizures, altered sensorium and corticalvisual loss with characteristic MRI features of subcortical white matter hyperintensities on T2W imaging. Though hypertension is a traditional hallmark of PRES, uncommon causes without hypertension have been described. We report an unusual such case without hypertension, precipitated by red cell transfusion.
Case Presentation:
A 40 years female received six units of packed cell transfusion for severe anemia (Hemoglobin 3.0 Gm %) transfused over 8 hours. She was having menorrhagia and was operated on for a hysterectomy. She developed a headache on the second postoperative day for five days and no cause was revealed in spite of extensive workup, including MRI brain and CSF study. On the sixth day, she had recurrent seizures followed by altered sensorium in the absence of hypertension. Her repeat MRI brain findings were consistent with PRES.
Conclusion:
We reviewed 25 cases, including the present one as PRES after the blood transfusion. 24 were females, and 16 of 19 developed PRES in the course of chronic anemia lasting over 1 month. Anemia was severe in 18 of 25 cases, with haemoglobin (Hb) levels < 4.0 Gm%. In 16 of 20 cases, Hb levels increased to 5 g/dl by red cell blood transfusion until the onset of PRES. On MRI brain, 23 of 25 cases showed vasogenic edema and 3 of 25 cases showed an irreversible neurological deficit. When treating severe chronic anemia rapid correction of Hb with blood transfusion, one should consider a possibility of PRES in post-transfusion neurological symptoms in spite of normal diagnostic workup.
1. INTRODUCTION
The Posterior Reversible Encephalopathy Syndrome (PRES) was first described by Hinchey et al. in 1996 [1-3]. Their clinical radiological syndrome was characterised by headache, seizures, altered sensorium and cortical visual disturbances with subcortical white matter potentially reversible permeability abnormalities revealed by unique MRI features on T2W and diffusion sequences [4-6]. This treatable condition is increasingly recognised due to its reversibility if treated early and due to the availability of MRI imaging [4-6]. It is usually associated with hypertension (HT), although there are few exceptions [7-9]. Eclampsia, renal injury, immunosuppressive drugs, autoimmune disease, stem cell transplantation, rejection of transplanted organs, TURP, sepsis, and shock are reported as risk factors for PRES in normotensive subjects [7-9]. However, few cases of PRES following blood or blood products transfusion have been reported [10-29].
The pathophysiology of PRES is still controversial, and it may differ in hypertension versus normotension circumstances [26, 30-39]. It is presumed that rapid correction of anemia resulted in an increase in total blood volume and viscosity, leading to cerebral blood volume overload with secondary vasoconstriction [26, 30-39]. This increase in cerebral perfusion exceeds cerebral autoregulation resulting in vasogenic or rarely cytotoxic edema of the brain [26, 30-39]. Also, chronic anemia per se may lead to endothelial dysfunction disrupting the integrity of the blood-brain barrier [26, 30-39]. Post blood transfusion PRES is a rare presentation and only 24 cases have been reported to date as per our knowledge.We report a unique case of PRES following massive blood transfusion for severe chronic anemia with normal initai MRI brain but later on showed the characteristic changes of the PRES. We have also reviewed previously published case reports of post-transfusion PRES including our case.
2. CASE REPORT
A 40 year female with a history of severe menorrhagia presented with lower abdominal pain, tiredness, and dyspnea on exertion to gynecology department before 6 days. Her ultrasound imaging of the pelvis revealed multiple large uterine fibroids. Her preoperative laboratory and clinical workup for major surgery were normal except for severe hypochromic microcytic anemia with hemoglobin of 3 Gm%. Her 3-month-old laboratory reports revealed Hb 2.5 Gm%, and she was on oral iron therapy followed by parenteral1000 mg ferrous carboxymaltose before 3 months. She received 6 units of packed cell volume (900 ml) transfusion over 8 hours on the day of admission. She was operated on for laparoscopic abdominal hysterectomy under spinal anesthesia on the next day.
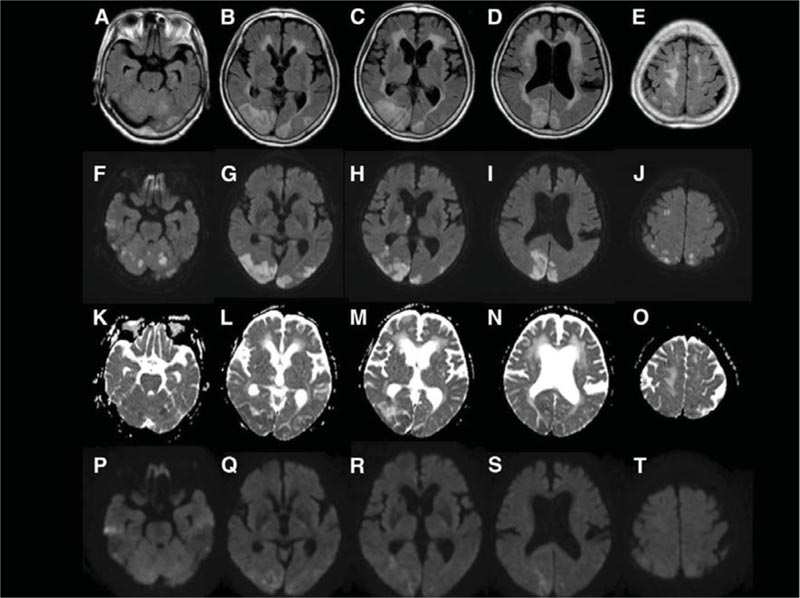
She had a mild generalized headache on the second postoperative day, which was considered as a spinal headache. Her headache continued on the third post operative day, which was diffuse and moderate in severity. Due to new-onset persistent headache, neurologist consultation was done. Her MRI brain and CSF study didn’t reveal any abnormality. On the 6th postoperative day, she had 6 episodes of generalized tonic clonic seizures over 5-6 hours. Each seizure episode lasted for 1-2 minutes associated with lateral margin tongue bite followed by a persistent altered mental status. Her vital signs were within normal range except mild tachycardia starting from the admission to the 6thpost operative day. She was referred to our center with temperature 98.8 F0, pulse 104 per minute and BP of 110/80 mm of Hg with normal respiration and 96% oxygen saturation on room air. The neurological assessment revealed an agitated patient who could not cooperate for diligent visual testing; her plantar responses were bilaterally extensor. She had no prior history of epilepsy, hypertension, or renal abnormalities or she was on any long-term medicines. She was admitted to ICU with a provisional diagnosis of status epilepticus. Other differentials were septic encephalomyelitis, mismatched blood transfusion and metabolic encephalopathies. She was treated with IV levetiracetam, midazolam infusion, cefoperazone and supportive treatment.
Her laboratory investigations revealed hemoglobin of 10 gm/dL with mild hypochromic microcytic anemia. Her RFT, LFT, blood sugar, arterial blood gas analysis and serum electrolytes were within normal limits. Her total serum proteins were 5.2 Gm %(Normal range: 6.5 to 8.5), and albumin was 3.0 (Normal range 3.5 to 4.5). Her thyroid and autoantibodies workup was negative. Her MRI Brain T2 and diffusion-weighted images performed on day six revealed white matter abnormalities suggestive of vasogenic edema in the bilateral parieto-occipital region consistent with PRES (Fig. 1). Her MR venogram was normal, but an MR angiogram was showing constriction of bilateral middle and posterior cerebral arteries. The lumbar puncture revealed normal CSF opening pressure. The CSF proteins, sugar, and cell counts were within normal limits. Electroencephalogram (EEG) showed a diffuse slowing in the theta range. She gradually improved over the next 4-5 days and her neurological examination was normal except for mild behavior abnormality. Her behaviour changes were considered as an adverse reaction to levetiracetam and the drug was replaced by diphenylhydantoin. She improved completely over the next 3-4 days and was discharged to home on day 15. Her repeat MRI brain with a diffusion study and angiogram after 6 weeks revealed complete resolution of white matter abnormalities and with normal intracranial vessels.
3. REVIEW OF THE LITERATURE
3.1. Methods
This review was based on a literature search by entering the keywords ‘PRES’ or ‘Posterior reversible encephalopathy’ and ‘blood transfusion’ in Medline (pubMed) database. The articles which were published in the English language from January 1997 to January 2019 were studied by the authors MVP, JKG, and ZM.
We identified 46 results by this search. We filtered 28 cases matching with our title and among them, 2 cases of sickle cell anemia and one case of liver transplant on immunosuppressants who developed post-BT PRES were excluded from the review because sickle cell anemia, organ transplant, and immunosuppressant drugs can trigger the PRES even in the absence of BT [33, 34].
4. RESULTS
The mean age was 44.48 (range 6 – 77 years), and the majority of the cases were females 24/25 (96%). Primary etiologies of anemia were menorrhagia of various causes 11 (44%), iron deficiency anemia 4(16%), aplastic anemia 2(8%), onco-surgical procedures 2(8%), thalassemia 1(4%), and gastrointestinal bleeding 2(8%).
Chronic anemia of more than one-month duration was more common 16/19 records (84.21%) as compared to the acute and subacute anemia 3/19 records(15.7%). The mean Hb value was 3.46 Gm% (1.4 -9.2) before the BT and 8.06 Gm% (7.5-13.8) after the BT. Anemia was severe in72.0% (18 of 25 cases), with hemoglobin levels < 4.0 Gm%. In 80% (16 of 20 recorded cases), hemoglobin levels increased to 5 g/dl by red cell blood transfusion until the onset of PRES. Chronic kidney injuries(CKI) with associated anemia were present in 12% (3of 25 cases).
The post-BT PRES neurological symptoms were manifested after 1 to 18 days (mean 6.04 days) of the transfusion in the form of encephalopathy in 10(40%), seizures in 16(64%), headache 17(68%), visual symptoms 12(48%) and focal deficits in 2 (8%). Hypertension (HT) was associated with fewer cases (40%), 10/25 of post-BT PRES in contrast to the previous studies of PRES from other aetiologies where HT was seen in more than 80% of patients. . The visual, motor, or cognitive deficit were persisted in 4/25 (16%) cases. The cerebral edema was vasogenic type in the majority of cases, 92% (23/25) on MRI brain in contrast to the cytotoxic edema revealed in only 8%(2 out of 25) cases. The vasoconstriction of cerebral vessels was identified in 48% (12/25) patients.
5. DISCUSSION
Most reported cases of PRES after the BT (96%) are females in contrast to the proportion of females in generalized PRES reports of about 60% [26, 30] (Table 1). The exact reasons for the higher proportion of post-BT PRES in females are not known; however, hormonal factors like low estrogen may be a contributing factor by promoting cerebral vasoconstriction [26, 30-39]. Previous reports showed an average age of post-BT PRES was perimenopausal, and a significant number of patients demonstrated vasoconstriction [10-29]. These findings support the low estrogen hypothesis of post-BT PRES (Table 2).
| S. No | Authors | Age/ Sex |
Etiology of Anemia | Course | Pre-BT Hb (Gm%) |
Post-BT Hb (Gm%) |
|---|---|---|---|---|---|---|
| 1 | Ito Y et al10 | 45/F | Uterine fibroids | Chronic | 2.0 | 10.0 |
| 2 | Boughammoura A et al [11] | 48/F | Uterine fibroids | Chronic | 3.0 | 8.0 |
| 3 | Heo K et al12 | 47/F | Aplasticanemia | Chronic | 1.5 | 10.9 |
| 4 | Kawano H et al.13 | 58/F | Onco surgery | NR | 7.7 | 10.9 |
| 5 | Kawano H et al13 | 77/F | Onco surgery | Acute | 9.2 | 13.3 |
| 6 | HuangYC et al14 | 32/F | Uterine fibroids | Chronic | 5.7 | 12.5 |
| 7 | GümüşH et al15 | 11/M | IDA | NR | NR | NR |
| 8 | SatoY et al16 | 42/F | CKI | Chronic | 5.7 | 11.7 |
| 9 | WadaKI et al17 | 56/F | Uteruscancer | Chronic | 2.0 | 9.2 |
| 10 | ZhaoZY et al18 | 28/F | Aplastic anemia | Chronic | 3.2 | 9.6 |
| 11 | ZhaoZY et al18 | 57/F | IDA | Chronic | 2.0 | 10.0 |
| 12 | DouYH et al19 | 50/F | DUB | Chronic | 1.5 | NR |
| 13 | DouYH et al19 | 46/F | Uterine fibroids | Chronic | 1.4 | NR |
| 14 | ShiraishiW et al20 | 36/F | DUB | Chronic | 1.4 | 11.3 |
| 15 | SarkarS et al21 | 6/F | Thalassemia | NR | 4.8 | NR |
| 16 | SinghK et al22 | 36/F | Uterine fibroids | Chronic | 1.7 | 8.8 |
| 17 | LiangH et al23 | 45/F | CKI | Chronic | 3.4 | 7.9 |
| 18 | LiangH et al23 | 47/F | CKI | Chronic | 3.0 | 10.4 |
| 19 | CevallosCA et al24 | 40/F | DU | Chronic | 3.1 | 8.6 |
| 20 | SudulaguntaSR et al25 | 35/F | Abortion | NR | 3.4 | 13.8 |
| 21 | Nakamura, Y et al26 | 75/F | GIbleeding | Subacute | 2.9 | 8.9 |
| 22 | H. Mitaka et al27 | 46/F | IDA | Chronic | 2.9 | 7.5 |
| 23 | Saito K et al28 | 64/F | GI Bleeding | Acute | 8.8 | NR |
| 24 | Suraj Kumar et al29 | 45/F | IDA | NR | 3.1 | 7.7 |
| 25 | Our case | 40/F | Uterine fibroid | chronic | 3.0 | 10.5 |
BT: Blood Transfusion
| S. No. | Volume of BT(ml) | Symptom onset after BT(days) | Clinical features | Brain edema | Vasoconstriction | Hypertension | Residual Deficit |
|---|---|---|---|---|---|---|---|
| 1 | 800 | 2 | S,H | VG | Yes | Yes | No |
| 2 | 1000 | 6 | E,S,F | VG | Yes | No | No |
| 3 | NR | 7 | E,S,H,V | VG | Yes | No | No |
| 4 | 1400 | 9 | E,S | VG | No | Yes | Yes |
| 5 | 2800 | 18 | E,S | VG | No | Yes | No |
| 6 | 1600 | 5 | H | VG | Yes | No | No |
| 7 | NR | NR | S,V | VG | No | No | No |
| 8 | 400 | 6 | S,H,V | VG | Yes | No | No |
| 9 | 2000 | 6 | E,S,V | VG | No | Yes | No |
| 10 | 1640 | 8 | H,V | VG | No | No | No |
| 11 | 1120 | 10 | S,H | VG | No | Yes | No |
| 12 | 3000 | NR | S,H | VG | Yes | Yes | No |
| 13 | 2500 | 15 | H,V | CX | Yes | Yes | No |
| 14 | 1120 | 12 | E,S | VG | No | No | Yes |
| 15 | 280 | 2 | E,H | VG | No | No | No |
| 16 | 560 | 2 | E,S,H,V | VG | No | Yes | No |
| 17 | 800 | 4 | H,V | VG | Yes | No | No |
| 18 | 750 | NR | S,H,V | VG | Yes | No | No |
| 19 | 840 | 4 | S,H,V,F | Vg | No | No | No |
| 20 | 700 | 10 | S | VG | No | No | No |
| 21 | 560 | 1 | E,V | CX | No | No | Yes |
| 22 | 900 | 14 | H, F | VG | Yes | Yes | No |
| 23 | 3000 | 1 | H E | V G | yes | Yes | |
| 24 | 900 | 7 | H, S, V | VG | No | No Yes No |
No |
| 25 | 900 | 2 | H, S | VG | yes | No |
The exact pathophysiology of the post-BT PRES is not known. However, a rapid rise in Hb level, viscosity, and volume of the blood is suggested to precipitate these subsets of PRES by previous studies [26, 40-42]. The chronic course of anemia itself can cause endothelial dysfunction and increase the velocity of cerebral blood flow [26, 42]. Previous reports showed a significant number of patients were having a chronic course of anemia in contrast to a very few patients of acute and subacute anemia [10-29], and this fact favors the theory of chronic anemia as a contributing factor of post-BT PRES. The sudden increase in blood viscosity and volume might trigger endothelial dysfunction and increase vascular resistance leading to extravasation of fluid and free radicals [26, 40-42]. CKI itself is a systemic inflammatory condition and its common association with hypertension may contribute to PRES triggering after BT [36]. Hypertension is a hallmark of the PRES by increasing vascular resistance and disturbing cerebral blood flow autoregulation [7]. Association of HT was seen in less than 50% of the post-BT PRES [10-29]. So, higher vascular resistance is not the sole mechanism, but other pathologies might be associated with it, and further studies are required to find out the exact pathophysiology. The post-BT PRES cases revealed vasogenic edema in most of the patients except the two cases whose MRI brain images revealed cytotoxic edema and the exact mechanism of this is not known [10-29].
Our patient was a female with an initial Hb level of 3.0 Gm % with a documented chronic course and hypoalbuminemia. She received an 1800 ml packed RBCs transfusion over 8 hours with a rise of 7.0 Gm % after the transfusion. She presented with a headache of 5 days followed by status epilepticus not associated with HT with normal MRI brain on the third day of headache. But due to a high index of suspicious, her MRI brain with contrast and MRA was repeated to know the cause of seizures with a persistent headache and it revealed bilateral parieto-occipital white matter lesions consistent with PRES. Her clinical recovery and resolution of MRI brain abnormal features confirmed our provisional diagnosis of PRES. Low serum albumin level reduces the osmotic pressure and may contribute to the aggravation of cerebral edema [26].
CONCLUSION
The massiveBT for the correction of severe chronic anemia may lead to PRES and it should be suspected if any patient develops neurological features after BT even in the absence of hypertension or normal initial neuroimaging, as prompt treatment may reduce or reverse the neurological sequalae.
CONSENT FOR PUBLICATION
We have obtained written consent from the patient for the publication of his case report in the biomedical journal.
FUNDING
None.
CONFLICT OF INTEREST
The authors declare no conflict of interest, financial or otherwise.
ACKNOWLEDGEMENTS
Declared none.


