All published articles of this journal are available on ScienceDirect.
Etiology, Risk Factors, Mortality and Morbidity of Status Epilepticus in Children: A Retrospective Cross-Sectional Study in Tehran, Iran
Abstract
Background:
Risk factors identification associated with status epilepticus is valuable in order to prevent morbidity and mortality in children. This study aimed to consider the etiology, risk factors, morbidity and mortality in children with status epilepticus.
Methods:
This retrospective cross-sectional study was performed on 119 patients aged from one month old to 15 years old. Patients’ data were recorded, including basic demographic, etiology and clinical information. The different risk factors correlated to morbidity and mortality were evaluated in this study.
Results:
The most common etiologies were acute symptomatic and febrile status epilepticus by 32.8% and 22.7%, respectively. Abnormal brain imaging results were reported far more frequently in patients with a history of neurodevelopmental delay and previous status epilepticus (p<0.001). The overall morbidity and mortality rates were 18.9% and 10.9%, respectively; while these rates in patients with delayed development (45.16% and 18.42%, respectively) were significantly higher than patients with normal development (8% and 7.4%, respectively). The morbidity rates in patients with previous seizures and previous status epilepticus were remarkably higher than those without previous history of seizure (26.41% vs 11.32%; p=0.047) and without previous status epilepticus (36.36% versus 14.28%; p=0.018). The length of hospital stay in patients with mortality was considerably longer than patients without mortality (12.30 ± 16.1 days vs 7.29 ± 6.24 days; p=0.033). The mortality rate in patients with normal Lumbar Puncture result was notably lower than those with abnormal LP result (2.9% vs 50%). The morbidity rate in patients with abnormal brain imaging results (p<0.001) was significantly greater than those in patients with normal results. The mortality rate was relatively higher in patients with abnormal imaging results compared to those normal results. Etiology was an important predictor of mortality and morbidity rates; acute symptomatic (32.8%), febrile status epilepticus (22.7%) and remote symptomatic (16.8%) etiologies were the most common underlying causes of S.E. While in children less than 3 years old, the acute symptomatic etiology and febrile status epilepticus etiologies were estimated as the most common, in most patients older than 3 years old the most common etiology of status epilepticus was unknown. Congenital brain defects etiology had the highest mortality (36.36%) and morbidity (42.85%) rate. The lowest morbidity (3.84%) and mortality (0%) rates were for patients with febrile status epilepticus etiology.
Conclusion:
Age, developmental delay, history of previous status epilepticus, the length of hospital stay, abnormal brain imaging results and the underlying etiology of status epilepticus were associated with increased morbidity and mortality among children with status epilepticus.
1. INTRODUCTION
Status epilepticus (SE) is a prevalent life-threatening condition in children which is defined according to ILAE (International League Against Epilepsy) as prolonged (longer than 5 minutes in generalized tonic-clonic convulsion, more than 10 minutes in focal seizures and greater than 20 minutes in absence seizures) continuous seizure activity or two or more sequential seizures without normal consciousness between them [1, 2]. Early diagnosis and initiation of treatment are crucial since SE can result in significant brain damage [3]. Generally, the incidence of SE is estimated at about 18-28 cases per 100,000 persons yearly [4, 5]. The prevalence of SE in the United States is 50,000-150,000 per year [6]. The incidence of SE is increasing in developing countries [7]. In a recent study in Iran, the prevalence of SE was 17.3% among children with seizures [8].
Status epilepticus can have devastating consequences like neuronal injuries, a decline in motor and cognitive skills and even death. This imposes considerable challenges to the health system. Negative effects of SE depend on the duration and type of seizures [5]. Generally, SE divides into two groups: convulsive and non-convulsive. There are four types of SE, including generalized, focal, non-convulsive and neonatal [8]. The most common form of SE is generalized tonic clonic (GTC), which is accompanied by the highest morbidity and mortality prevalence among children [9]. Most episodes of SE happen in the absence of prior seizure history. In the general population, infants and the elderly are the groups at higher risk for developing SE. Recent data reveals that approximately 10-20% of children with epilepsy will develop one episode of SE [10, 11].
One of the most important issues regarding the management of seizure in children is to identify the etiology associated with SE regarding the prevention of disease and reduction in its morbidity and mortality rates [12]. In adults, some known etiologies are reported as fever, cerebral infections, electrolyte imbalance, anti-convulsive drug withdrawal, cerebrovascular disease, trauma and congenital anomalies. The etiology and risk factors of SE are less identified in pediatrics [13]. Thus, identification of etiology and the most prevalent risk factors for SE in children can help us to decrease SE related morbidity and mortality. The purpose of recent research is to investigate associated risk factors and etiologies of SE and correlation with morbidity and mortality in the Iranian pediatric age group.
2. MATERIALS AND METHODS
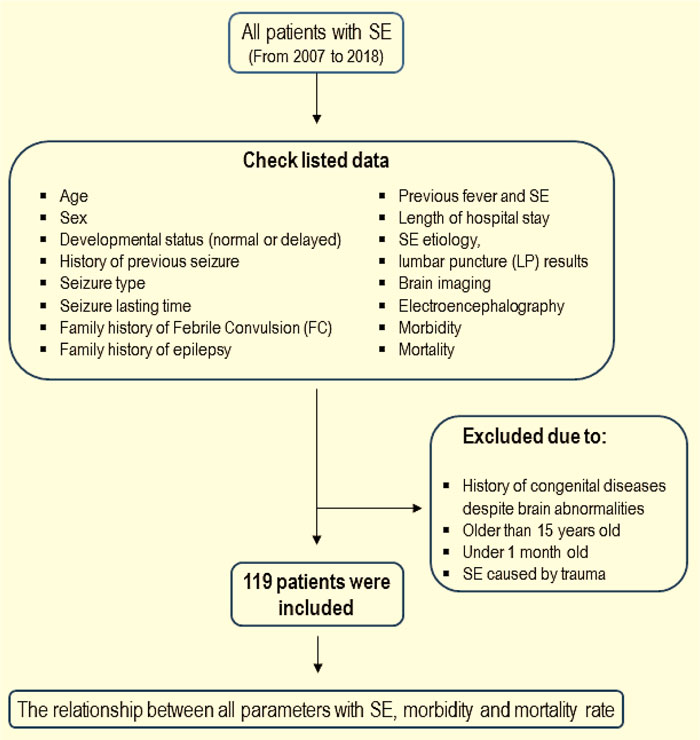
In this retrospective and cross-sectional study, all patients with SE aged from one month old to 15 years old admitted to Hazrat-e Rasool Hospital (Tehran, Iran) were enrolled in this study between the years 2007 to 2018. Fig. (1) is illustrating the process of patients’ selection. The study was approved by the Ethics Committee of Iran University of Medical Sciences (IR.IUMS.FMD.REC.1398.105). At the beginning of the study, a checklist was prepared in order to collect information on demographic and basic clinical data, including age, sex, developmental status (normal or delayed), history of previous seizure, seizure type (focal or generalized), duration of the seizure (over 60 minutes and less than 60 minutes), family history of febrile convulsion (FC), family history of epilepsy, history of previous SE, length of hospital stay, etiology (febrile status epilepticus, acute symptomatic, remote symptomatic, congenital brain abnormalities, unknown), lumbar puncture results (normal or abnormal), brain imaging (CT scan and MRI), electroencephalography (EEG), and the morbidity and mortality rates following status epilepticus. Any abnormality in CT scan or MRI, including congenital or acquired, was recorded as abnormal imaging results. Patients with a history of congenital diseases despite brain abnormalities, aged older than 15 years old or less than one-month old and SE caused by trauma, were excluded from the present study. According to the inclusion and exclusion criteria, 119 patients were enrolled in this research. The checklists were completed according to medical records and information achieved by patients’ follow-ups. The relationship between all parameters and status epilepticus, morbidity and mortality rates was considered in these patients.
3. STATISTICAL ANALYSIS
All quantitative data were analyzed using the descriptive program and presented as Mean ± SD. Crosstabs and Chi-Square tests were used to compare parameters between two groups. The comparison of the mean in parametric data between two groups was analyzed using an independent student sample t-test. In this study, a p-value of less than 0.05 was considered statistically considerable. The SPSS software (IBM, version 19) was applied for data analysis.
4. RESULTS
A total of 119 cases with a mean age of 45.05 ± 40.37 months were enrolled in the study. The results of the basic demographic and clinical findings of all cases are summarized in Table 1. The percentage of patients less than 3 years old and older than 3 years old was 50.4% (60 cases) and 49.6% (59 cases), respectively. Of all patients, 68 patients (57.1%) were male and 51 cases (42.9%) were female. There was no remarkable difference in the mean age of patients between males and females (41.47 ± 40.6 vs 49.83 ± 39.95 months-old; p=0.26). The medical history of all patients revealed that 32.2% (38 cases) had delayed development. The prevalence of delayed development in boys and girls was 32.35% (22 cases) and 31.37% (16 cases), respectively (p=0.91). The prevalence of previous seizure and SE was 50.4% and 20.2%, respectively. Moreover, 70 patients (58.8%) had SE with fever. Compared to focal seizure found in 21.1% of cases, the generalized form was the most common type of seizure among patients (78.9%). The percentage of patients with a family history of FC and epilepsy was 11.8% and 21.0%. Acute symptomatic and febrile status epilepticus were the first and second most common etiologies by 32.8% and 22.7%, respectively. There was no significant difference in the frequency of these parameters between males and females.
A considerable difference was found among the etiology between patients in two age groups (Table 2; p<0.001). While acute symptomatic etiology was the highest cause of SE occurrence in patients less than 3 years old (38.33%), thus the highest prevalent etiology of SE in patients aged over 3 years old was unknown (30.50%). The prevalence of febrile status epilepticus in children aged less than 3 years old (28.33%) was approximately two times more than patients aged over 3 years old (16.94%).
| Variables | Results |
|---|---|
| Age (Month) | 45.05 ± 40.37 |
| Age groups | |
| >3 years (%) | 59 (49.6%) |
| <3 years (%) | 60 (50.4%) |
| Gender | |
| Boys (%) | 68 (57.1%) |
| Girls (%) | 51 (42.9%) |
| Developmental history | |
| Normal (%) | 81 (67.8%) |
| Delayed (%) | 38 (32.2%) |
| Previous seizure | |
| Yes (%) | 60 (50.4%) |
| No (%) | 59 (49.6%) |
| Seizure type | |
| Focal (%) | 29 (21.1%) |
| Generalized (%) | 90 (78.9%) |
| Seizure lasting (minute) | 42.58 ± 37.73 |
| Previous status | |
| Yes (%) | 24 (20.2%) |
| No (%) | 95 (79.8%) |
| Status with fever | |
| Yes (%) | 70 (58.8%) |
| No (%) | 49 (41.2%) |
| Family history of FC | |
| Yes (%) | 14 (11.8%) |
| No (%) | 105 (88.2%) |
| Family history of epilepsy | |
| Yes (%) | 25 (21.0%) |
| No (%) | 94 (79.0%) |
| Hospital stay (day) | 8.0 ± 7.85 |
| Etiology | |
| Status F.C | 27 (22.7%) |
| Acute symptomatic | 39 (32.8%) |
| Remote symptomatic | 20 (16.8%) |
| Congenital brain defects | 11 (9.2%) |
| Unknown | 22 (18.5%) |
| LP | |
| Normal | 69 (58.0%) |
| Abnormal | 2 (1.7%) |
| No provided | 48 (40.3%) |
| Age group | N | p-value | |||
|---|---|---|---|---|---|
| Under 3 years | Over 3 years | ||||
| Etiology | Status F.C | 17 (28.33%) | 10 (16.94%) | 27 | <0.001 |
| Acute symptomatic | 23 (38.33%) | 16 (27.11%) | 39 | ||
| Remote symptomatic | 6 (10.0%) | 14 (23.72%) | 20 | ||
| Congenital brain defects | 10 (16.66%) | 1 (1.69%) | 11 | ||
| Unknown | 4 (6.66%) | 18 (30.50%) | 22 | ||
Brain imaging (CT scan, MRI) and electroencephalo- graphy (EEG) were not performed in about 40% of patients (51.3% for EEG, 38.7% for CT scan and 65.5% for MRI). The prevalence of normal results reported in patients undergone imaging tests, including EEG, CT scan and MRI, was 34.5%, 43.7% and 18.5%, respectively. A substantial difference was observed in brain imaging and EEG results of patients with normal and delayed development (Table 3). Compared to patients with delayed development, patients with normal developmental history had a significantly higher rate of normal EEG (84.09% vs 28.57%; p<0.001), CT scan (87.27% vs. 22.22%; p<0.001) and MRI (72.41% vs. 8.33%; p<0.001) results.
A comparison between brain imaging and EEG in patients with and without a history of previous SE is shown in Table 4. Although the nonsignificant difference was found in brain imaging results and EEG tests between the two groups, patients without a history of previous SE had a relatively higher prevalence of normal results of EEG (72.91% vs. 60.0%), CT scan (74.6% vs. 50%) and MRI (58.06% vs. 40.0%) compared to those with a history of SE.
The morbidity and mortality rates in all patients were 18.9% (20 cases) and 10.9% (13 cases), respectively. There was no substantial difference in morbidity (p=0.66) and mortality (p=0.35) rates between male (20.33% and 33.89%, respectively) and female groups (17.02% and 34.04%, respectively). Correlation between mortality and morbidity rates with basic demographic and clinical characteristics of patients is shown in Table 5. While the mean age of patients with morbidity was notably higher than those without morbidity (59.27 ± 43.47 months vs. 41.32 ± 36.91 months; p=0.028), the mortality rate was significantly higher in patients with lower mean age compared to those with a higher mean age (Table 5). The morbidity and mortality rates in patients with delayed development (45.16% and 18.42%, respectively) were considerably higher than in patients with normal development (8% and 7.4%, respectively). The morbidity rate in patients with previous seizures was meaningfully higher than those without previous seizures (26.41% vs. 11.32%; p=0.047). Furthermore, the morbidity rate in patients with previous SE was far greater than those without previous SE (36.36% vs. 14.28%; p=0.018). The length of hospital stay in patients with mortality was considerably longer than patients without mortality (12.30 ± 16.1 days vs. 7.29 ± 6.24 days; p=0.033). The mortality rate in patients with normal LP results was notably lower than those with abnormal LP results (2.9% vs. 50%).
Correlation between mortality and morbidity rates with brain imaging and EEG results indicates that the morbidity rate in patients with abnormal EEG (37.5% vs. 7.14%; p=0.022), CT scan (50% vs. 3.92%; p<0.001) and MRI (88.23% vs. 0%; p<0.001) findings was meaningfully greater than those in patients with normal results. Although the mortality rate was relatively higher in patients with abnormal EEG (5.88% vs. 0%), CT (14.28% vs. 7.69%) and MRI (10.52% vs. 4.54%), these differences were not statistically significant (Table 6).
| Imaging type | Development | N | p-value | ||
|---|---|---|---|---|---|
| Normal | Delayed | ||||
| EEG | Normal | 37 (84.09%) | 4 (28.57%) | 41 | <0.001 |
| Abnormal | 7 (15.9%) | 10 (71.42%) | 17 | ||
| CT scan | Normal | 48 (87.27%) | 4 (22.22%) | 52 | <0.001 |
| Abnormal | 7 (12.72%) | 14 (77.77%) | 21 | ||
| MRI | Normal | 21 (72.41%) | 1 (8.33%) | 22 | <0.001 |
| Abnormal | 8 (27.58%) | 11 (91.66%) | 19 | ||
| Imaging type | History of the previous status | N | p-value | ||
|---|---|---|---|---|---|
| Yes | No | ||||
| EEG | Normal | 6 (60.0%) | 35 (72.91%) | 41 | 0.55 |
| Abnormal | 4 (40.0%) | 13 (27.08%) | 15 | ||
| CT scan | Normal | 5 (50%) | 47 (74.6%) | 52 | 0.11 |
| Abnormal | 5 (50%) | 16 (25.39%) | 21 | ||
| MRI | Normal | 4 (40.0%) | 18 (58.06%) | 22 | 0.33 |
| Abnormal | 6 (60.0%) | 13 (41.93%) | 18 | ||
| Variables | Morbidity | p-value | Mortality | p-value | ||
|---|---|---|---|---|---|---|
| Yes | No | Yes | No | |||
| Age (Month) | 59.27 ± 43.47 | 41.32 ± 36.91 | 0.028 | 23.15 ± 33.86 | 47.41 ± 39.98 | 0.038 |
| Developmental history | ||||||
| Normal (%) | 6 (8.0%) | 69 (92.0%) | <0.001 | 6 (7.4%) | 75 (92.59%) | 0.073 |
| Delayed (%) | 14 (45.16%) | 17 (54.83%) | 7 (18.42%) | 31 (81.57%) | ||
| Previous seizure | ||||||
| Yes (%) | 14 (26.41%) | 39 (73.58%) | 0.047 | 7 (11.66%) | 53 (88.33%) | 0.79 |
| No (%) | 6 (11.32%) | 47 (88.67%) | 6 (10.16%) | 53 (89.83%) | ||
| Seizure type | ||||||
| Focal (%) | 6 (25.0%) | 18 (75.0%) | 0.38 | 2 (7.69%) | 24 (92.3%) | 0.55 |
| Generalized (%) | 14 (17.07%) | 68 (82.92%) | 11 (11.82%) | 82 (88.17%) | ||
| Seizure lasting (minute) | 51.45 ± 28.69 | 39.16 ± 40.76 | 0.2 | 50 ± 27.71 | 42.06 ± 38.80 | 0.49 |
| Seizure lasting | ||||||
| <60 minutes | 19 (20.22%) | 71 (79.77%) | 0.88 | 11 (11%) | 89 (89%) | 0.92 |
| >60 minutes | 1 (22.22%) | 7 (77.77%) | 2 (10%) | 9 (90%) | ||
| Previous status | ||||||
| Yes (%) | 8 (36.36%) | 14 (63.63%) | 0.018 | 2 (8.33%) | 22 (91.66%) | 0.64 |
| No (%) | 12 (14.28%) | 72 (85.71%) | 11 (11.57%) | 84 (88.42%) | ||
| Status with fever | ||||||
| Yes (%) | 10 (16.39%) | 51 (83.60%) | 0.44 | 9 (12.85%) | 61 (87.14%) | 0.41 |
| No (%) | 10 (22.22%) | 35 (77.77%) | 4 (8.16%) | 45 (91.83%) | ||
| Family history of FC | ||||||
| Yes (%) | 2 (14.28%) | 12 (85.71%) | 0.63 | 0 | 14 (100%) | 0.16 |
| No (%) | 18 (19.56%) | 74 (80.43%) | 13 (12.38%) | 92 (87.61%) | ||
| Family history of epilepsy | ||||||
| Yes (%) | 7 (29.16%) | 17 (70.83%) | 0.14 | 1 (4.0%) | 24 (96.0%) | 0.21 |
| No (%) | 13 (15.85%) | 69 (84.14%) | 12 (12.76%) | 82 (87.23%) | ||
| Hospital stay (day) | 7.57 ± 7.45 | 7.25 ± 5.93 | 0.9 | 12.30 ± 16.1 | 7.29 ± 6.24 | 0.033 |
| Etiology | ||||||
| Status F.C | 1 (3.84%) | 26 (96.29%) | <0.001 | 0 | 27 (100%) | 0.015 |
| Acute symptomatic | 6 (18.18%) | 27 (81.81%) | 6 (15.38%) | 33 (84.61%) | ||
| Remote symptomatic | 3 (16.16%) | 15 (83.33%) | 2 (10%) | 18 (90%) | ||
| Congenital brain defects | 3 (42.85%) | 4 (57.14%) | 4 (36.36%) | 7 (63.63%) | ||
| Unknown | 7 (33.33%) | 14 (66.66%) | 1 (4.54%) | 21 (95.45%) | ||
| LP | ||||||
| Normal | 9 (13.43%) | 58 (86.56%) | 0.69 | 2 (2.98%) | 67 (97.1%) | 0.001 |
| Abnormal | 0 (0%) | 4 (100%) | 1 (50%) | 1 (50%) | ||
| Variables | Morbidity | p-value | Mortality | p-value | ||
|---|---|---|---|---|---|---|
| Yes | No | Yes | No | |||
| EEG | ||||||
| Normal (%) | 3 (7.14%) | 39 (92.85%) | 0.022 | 0 | 41 (100%) | 0.11 |
| Abnormal (%) | 6 (37.5%) | 10 (62.5%) | 1 (5.88%) | 16 (94.11%) | ||
| CT scan | ||||||
| Normal (%) | 2 (3.92%) | 49 (96.07%) | <0.001 | 4 (7.69%) | 48 (92.3%) | 0.38 |
| Abnormal (%) | 11 (50.0%) | 11 (50.0%) | 3 (14.28%) | 18 (85.71%) | ||
| MRI | ||||||
| Normal (%) | 0 | 24 (100%) | <0.001 | 1 (4.54%) | 21 (95.45%) | 0.46 |
| Abnormal (%) | 15 (88.23%) | 2 (11.76%) | 2 (10.52%) | 17 (89.74%) | ||
5. DISCUSSION
In this research, we considered the etiology and associated risk factors of morbidity and mortality in children with status epilepticus. We did not find a significant difference in S.E prevalence between male and female groups. The prevalence of S.E in male and female children was 57.1% and 42.9%, respectively. Overall, acute symptomatic (32.8%), febrile status epilepticus (22.7%) and remote symptomatic (16.8%) etiologies were the most common underlying causes of S.E. While in children less than 3 years old, the acute symptomatic etiology and febrile status epilepticus etiology were estimated as the most common causes of S.E. The etiology of S.E in most patients older than 3 years old was unknown. Acute symptomatic etiology of SE was the second common cause of disease in cases older than 3 years old. Our study revealed the importance of etiology in predicting mortality and morbidity rates following SE, with the highest risk of morbidity (42.85%) and mortality (36.36%) in patients with congenital brain defects compared to the lowest morbidity (3.84%) and mortality (0%) in patients with febrile status epilepticus etiology. Cherian et al. [14] reported that infection (35.7%), change in antiepileptic drugs (20%), idiopathic (9%), metabolic disorders (8%), brain infection (6%) and trauma (3.5%) were the most prevalent causes of S.E in children. Kang et al. [15] reported that the most common cause of S.E in children was unknown (40.7%), which was followed by febrile conditions (35.4%), seizure (29.1%), brain infection (5.3%), metabolic disorders (4.2%), anoxia (3.2%), and trauma (0.5%). Riviello et al. [16] demonstrated that remote symptomatic (27%), fever (17%), cryptogenic (14%), brain abscess (11%), and metabolic disorders (5%) were the leading causes of SE.
Our findings revealed that the morbidity and mortality rates in these patients were 18.9% and 10.9%, respectively. Similarly, Logroscino et al. [17] reported that the mortality rate of children with S.E was 10%, while these results were consistent with the findings of our study. Previous studies reported the mortality rate of children with SE from 3.45% to 31.75% [18-19]. Therefore, it is necessary to consider the etiology and role of different factors in the evaluation of prognosis, mortality and morbidity in these patients. Recent researches have revealed the leading role of various factors in prognosis, morbidity and mortality in children with S.E. Hocker et al., 6 reported that long-lasting mechanical ventilation was considered an important risk factor in the rate of mortality among children with SE. In another survey, Ristić et al. [20] concluded that there was a notable and positive correlation between the length of status and mortality rate in these cases. Claassen et al. [21] found that female gender, hypoxic-ischemic brain injury and a higher rate of comorbidity were important risk factors in the prevalence of mortality in patients with SE. Logroscino et al. [17] reported that generalized myoclonic seizures, refractory seizures, and acute symptomatic were the most important risk factors leading to increased mortality in children with SE. In our study, acute symptomatic, febrile status epilepticus and remote symptomatic etiologies were the most common etiologies for SE; however, the morbidity and mortality rates in patients with congenital brain defects were higher compared to other types.
In this study, we also found that age, previous history SE, length of hospital stay, LP result, and developmental status were the most important factors in the evaluation of prognosis in mortality and morbidity among these patients. Although only two patients had abnormal LP results, further investigation is required in assessing this etiology. Our data demonstrated that the morbidity rate of older patients was remarkably higher than younger children, while the mortality rate in younger cases was significantly higher than older children. In a previous study, Koubeissi et al. [18] reported that there was a meaningful and positive correlation between age and mortality rate in patients with SE. These data indicate the importance of age as a possible risk factor in morbidity and mortality rates of patients with SE. Developmental status was another important factor for the prognosis of morbidity and mortality in children with S.E. In our study, 32.2% of patients had abnormal development. Patients with abnormal development not only had substantially higher morbidity and mortality rate compared to those with normal development, but also they had more prevalent abnormal EEG, CT and MRI results. Therefore, it appears that abnormal development of children may be a risk factor for SE incidence, increased risk of brain damage and subsequently higher morbidity and mortality rate in these patients.
Some studies demonstrated that 40-60% of patients with SE had no history of previous seizures [17, 18]. Kang et al. [15] revealed that 71% of patients under 15 years old had no history of previous seizures. In contrast, Scholtes et al. [3] reported that 68% of children with SE had a history of previous seizures. In another study, DeLorenzo et al. [22] found that 58% of children with SE had no history of seizure. Similar to a study by DeLorenzo, about 50% of our patients had no history of previous seizures. Nevertheless, we found a significant relationship between morbidity and history of previous SE and seizure in these patients. The prevalence of morbidity in children with previous SE and seizure was much higher than those without a previous history. These data indicate that although a history of previous seizures might not be correlated to the incidence of SE in children, it can increase the risk of morbidity and mortality rate in these patients, which needs to be considered.
Our data also revealed a considerable correlation between morbidity and mortality with the length of hospital stay. The length of hospital stay in patients with mortality and morbidity was remarkably higher than those without morbidity and mortality. Similarly, several studies reported a significant relationship between the length of hospital stay with morbidity and mortality rate in these patients [23, 24]. Our study revealed that the brain imaging results were valuable in the evaluation of prognosis, morbidity and mortality in these patients. Patients with abnormal EEG, CT and MRI findings had higher morbidity and mortality rate compared to those with normal imaging results. Therefore, our data indicate the importance of age, previous SE, LP results, brain imaging and EEG findings as possible risk factors for morbidity and mortality among patients with S.E.
CONCLUSION
The results of this study showed that the highest prevalent etiologies in all patients were correlated to acute symptomatic, febrile status epilepticus and remote symptomatic etiology. Various risk factors such as age, abnormal development, history of previous SE, length of hospital stay, abnormal LP results, abnormal brain imaging and EEG results were associated with increased morbidity and mortality in children with SE. Therefore, evaluation of these risk factors can be helpful in the management, prognosis, evaluation of mortality and morbidity in children with SE seizures.
AUTHORS' CONTRIBUTIONS
VM and SH designed and managed the study. SE and RA analyzed and interpreted patients’ data and performed follow-ups in patients. VM and SE were major contributors to writing the manuscript. All of the authors read and approved the final manuscript.
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
This study was approved by the Ethics Committee of Iran University of Medical Sciences. Ethical number was IR.IUMS.FMD.REC.1398.105.
HUMAN AND ANIMAL RIGHTS
Not applicable.
CONSENT FOR PUBLICATION
The consent for publication of personal detailed data was obtained from parents.
AVAILABILITY OF DATA AND MATERIALS
The datasets used during the current study are available in the manuscript.
FUNDING
None.
CONFLICT OF INTEREST
The authors declare no conflict of interest, financial or otherwise.
ACKNOWLEDGEMENTS
The authors would like to appreciate the staff at Hazrat-Rasool Hospital for their technical assistance and also appreciate the Aliasghar Clinical Research Development Center, Iran University of Medical Sciences. This study was a pediatric residency thesis by Dr. Elaheh Soltanieh. They also appreciate Vice Chancellor for Research of Iran University of Medical Sciences.


