All published articles of this journal are available on ScienceDirect.
Efficacy of VNS for Drug-Resistant Epilepsy in Structural Brain Lesions
Abstract
Background:
Vagus nerve stimulation (VNS) has been used for the treatment of drug-resistant epilepsy, especially in patients who are not candidates for surgical intervention. In fact, it was approved by the US FDA in 1997 as an adjunctive treatment for medically intractable epilepsy.
Objective:
In this study, we investigated the efficacy of VNS in drug-resistant epilepsy associated with structural brain lesions (SBLs).
Methods:
We retrospectively analyzed the effect of VNS on 25 patients diagnosed with intractable epilepsy-associated SBL, and compared the results to 19 patients with intractable epilepsy and normal neuroimaging. All patients underwent VNS insertion at the National Neurosciences Institute, King Fahad Medical City (Riyadh, Saudi Arabia) between 2008 and 2018.
Results:
The response rate (RR) for patients with drug-resistant epilepsy-associated SBL was 24% after 3 months, 36% after 6 months, and 48% after 1 year, reaching 76% over time. The mean follow-up period was 63.3 months. For non-SBL patients, the RR was 10.5% after 3 months, 36.8% after 6 months, and 47.4% after 1 year, reaching 73.7% over time. The mean follow-up period was 59.2 months. There was no statistically significant difference between the two groups regarding RR, VNS settings, and other parameters, including anti-epileptic drug use and demographics data.
Conclusion:
VNS is strongly considered for intractable epilepsy in SBL patients, especially if they are not candidates for surgical intervention. Over time, those patients will receive increased benefits from VNS therapy.
1. INTRODUCTION
Epilepsy is one of the most common and disabling neurological diseases, affecting about 50 million people worldwide [1], with a prevalence of 6.54 per 1000 in Saudi Arabia [2].
It was proposed by the International League Against Epilepsy and International Bureau for Epilepsy in 2005, as a brain disorder characterized by an enduring predisposition to generate epileptic seizures and by the neurobiological, cognitive, psychological, and social consequences of this condition [3]; however, a new practical clinical definition of epilepsy has now been agreed upon. Epilepsy is defined as any one of the following conditions: (a) at least two unprovoked (or reflex) seizures occurring >24 hours apart; (b) one unprovoked (or reflex) seizure and a probability of further seizures (at least 60%), occurring over the next 10 years; or (c) diagnosis of an epilepsy syndrome [4]. It is characterized by a long-term risk of recurrent seizures, which may present in several ways depending on the part of the brain involved and the person age [5]. The management of epilepsy mainly depends on the medication, and approximately 70–80% of patients have well-controlled disease [6-10].
However, patients who have breakthrough seizures despite treatment with two or more anticonvulsant medications are generally considered to have drug-resistant epilepsy; thus, other therapeutic options can be considered, including epilepsy surgery, diet, and electrical stimulation, such as vagus nerve stimulation (VNS) [11].
VNS was first approved by the US Food and Drug Administration in 1997 as adjunctive therapy for reducing the frequency of seizures in patients > 12 years with partial-onset seizures refractory to antiepileptic drugs (AEDs] [12]. Since then, VNS has shown a favorable outcome for intractable focal, focal-to-bilateral tonic-clonic epilepsy, and generalized onset epilepsy, including atonic seizures in adults [13-16], and compared to them, VNS has shown similar efficacy and safety outcomes in children [17-20]. In addition, epileptic syndromes such as Lennox-Gastaut syndrome, Rett syndrome, epilepsy-related comorbidities, hypothalamic hamartoma, and tuberous sclerosis (TS) complex have shown a good response to VNS insertion [21-25].
VNS also has other effects, including improvements in attention, alertness, and psychomotor activity [19]. In 2005, VNS was approved for treatment-resistant depression in patients ≥18 years old with at least one major depressive episode defined by the Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition [26]. Recently, VNS studies have shown favourable outcomes in patients with cluster headaches and migraines [27]. And secondary to its anti-inflammatory effects, VNS has shown success in treating inflammatory disorders such as rheumatoid arthritis, diabetes, sepsis, and cardiovascular diseases [28]. Additional studies are needed to investigate other applications of VNS in various diseases.
The mechanism underlying the effects of VMS is not fully understood; however, some proposed theories include anti-inflammatory effects and changes in monoamines [29, 30].
The most common acute side effects are infection, vocal cord paresis, and lower facial nerve palsy, whereas cardiac-related adverse effects are bradycardia and asystole, which mainly occurred during device testing [31, 32]. For long-term use, the most common side effects are stimuli-related cough, throat pain, and hoarseness, all of which tend to improve over time. Side effects appear to be similar in children and adults [33].
Numerous studies have supported VNS therapy for medically intractable epilepsy [34-38]. However, patient characteristics predictive of responsiveness to VNS therapy remain unknown, and an association between responsiveness to VNS therapy and certain patient features has not been consistently shown [39, 40].
The main objectives of this study were to evaluate the effectiveness of VNS in drug-resistant epilepsy associated with structural brain lesions (SBLs), in order to define a clear association between VNS therapy response and SBLs, although the main treatment in such cases depends on the surgery, however, some patients are still not a candidate for surgery, or the risk of seizures will still remain even after surgery.
2. METHODS AND MATERIALS
We retrospectively analyzed patients with drug-resistant epilepsy and SBLs, who underwent VNS insertion at the National Neurosciences Institute, King Fahad Medical City (KFMC, Riyadh, Saudi Arabia) between 2008 and 2018. The outcome of VNS in patients with drug-resistant epilepsy and SBLs was compared to those without SBLs to determine the VNS efficacy. A case-control study design was utilized that matched the two groups. Patients were excluded if there were no data for the last follow-up visit.
No single set of guidelines was used to choose patients suitable for VNS implantation; however, all patients were admitted to the epilepsy monitoring unit for evaluation and pre-surgical assessment, evaluated by an epileptologist, and discussed in the Epilepsy Surgery Meeting.
Stimulation was initiated when the patient had fully recovered, usually 1 ± 4 weeks after surgery. The stimulation settings were modified at the discretion of the treating epileptologist during follow-up visits.
Patient demographics were documented, including sex, age, age at epilepsy onset, epilepsy duration, age of insertion of VNS, duration of epilepsy before VNS insertion, number of AEDs before and after VNS insertion, prior epilepsy surgery, magnetic resonance imaging (MRI) of the brain lesion including type and location, epilepsy type (focal or generalized), comorbidities, VNS insertion date, model, latest clinic VNS setting, seizure baseline, side effects, follow-up response at 3–6 months, 1 year, and every year thereafter, total follow-up duration, year of VNS discontinuation and reasons, and average VNS battery life. The change in seizure frequency after placement of VNS was based on patient diaries and epilepsy clinic chart review.
Based on post-VNS seizure reduction, the patients were graded at the follow-up visits as non-responder (no seizure frequency reduction or <50% reduction from baseline) and responder (seizure frequency reduction with >50% from baseline). The responder categories were: R50 if seizure frequency reduction from baseline was >50% and <75% and R75 if seizure frequency reduction was >75% from baseline and patients were seizure-free (SF, complete seizure freedom without aura).
The Student’s t-test was used to compare the means of continuous variables, and the Fisher’s exact test/chi-squared tests were used for categorical variables. The data were entered into a database and analyzed using Statistical Analysis Software. Statistical analysis tests for both continuous and categorical variables were used as appropriate. No other systems were used. We calculated the measures of association between variables, which were expressed as the p-value. For each test, the level of significance was set at 0.05.
3. RESULTS
This study included 44 patients, who underwent VNS implantation at KFMC between November 2008 and April 2018.
Group 1 comprised 25 patients with SBLs; 14 were males (56%) and 11 were females (44%), with a mean age of 25.8 years (standard deviation [SD] 11.8, range: 11–53). The mean age at seizure onset was 5.3 years (SD 5.6, range: 1–26), and the mean age at VNS implantation was 18.8 years (SD 11.9, range: 3–47). The mean epilepsy duration was 20.5 years (SD 10, range: 9–50). Three patients (12%) had prior epilepsy surgery before VNS implantation, including corpus colostomy, tumour resection, and one patient had intracranial electro-encephalography (EEG) recording without subsequent resection. The mean number of AEDs before VNS was 3.2 (range: 1–5), and the mean number of AEDs after VNS was 3.2 (range: 1–5). Neuroimaging showed the following: encephalo-malacia/gliosis in 10 patients (40%), focal cortical dysplasia (FCD) in 5 patients (20%), mesial temporal sclerosis (MTS) in 5 patients (20%), vascular insult in 1 patient (4%), heterotopia in 2 patients (8%), tumour in 1 patient (4%), and TS in 1 patient (4%). The neuroimaging details are shown in Table 1. Some patients underwent surgery first but developed seizures later, some were offered surgical intervention but refused, and others were not good candidates for surgery.
| Patient Number | Brain MRI Results |
|---|---|
| 1st | Left temporal lobe FCD |
| 2nd | Left frontal ganglioma |
| 3rd | Bilateral encephalomalacia and gliosis |
| 4th | Bilateral frontal encephalomalacia. |
| 5th | Left occipital FCD |
| 6th | Left frontoparietal encephalomalacia and gliosis |
| 7th | Right orbito-frontal FCD |
| 8th | Multifocal bilateral cerebral encephalomalacia |
| 9th | Left MTS |
| 10th | Right MTS |
| 11th | Left temporal FCD |
| 12th | Subependymal cortical heterotropia |
| 13th | Bilateral encephalomalacia and gliosis |
| 14th | Right MTS |
| 15th | Bilateral hemisphere encephalomalacia and gliosis |
| 16th | TS: cortical tubers + subependymal nodular lesions |
| 17th | Subependymal nodular gray matter heterotopia |
| 18th | Bilateral frontal encephalomalacia and gliosis |
| 19th | Left occipital vascular insult |
| 20th | Bilateral encephalomalacia and gliosis |
| 21th | Left hemisphere encephalomalacia. |
| 22th | Left temporal FCD |
| 23th | Bilateral MTS |
| 24th | Right MTS |
| 25th | Right temporoparietal encephalomalacia and gliosis |
Regarding the epilepsy characteristics, 12 patients (48%) had generalized seizures and 13 (52%) had focal seizures. Regarding seizure frequency, it was daily in 17 patients (68%), weekly in 7 patients (28%), and monthly in 1 patient (4%). Eighteen patients (72%) had other comorbidities; in fact, 10 patients (40%) had developmental delay, 7 (28%) had cognitive impairment, 1 (4%) had attention deficit hyperactivity disorder, and 1 (4%) had intellectual disabilities. For VNS settings, Model 102 was used in 2 patients (8%), Model 103 in 13 patients (52%), and Model 106 in 10 patients (40%). VNS parameters at last follow-up are shown in Table 2.
Fourteen patients had average battery usage of 5.5 years (range: 2–7). Side effects were reported in eight patients (32%), including hoarseness, cough, shortness of breath, and wound infection. VNS was discontinued in three patients (12%), due to side effects in two and because of no improvement in one. Regarding the outcome and latest follow-up period, 19 patients were responders (76%): 6 were R50 (31.5%) and 13 were R75 (68.4%); 6 patients (24%) were non-responders. The mean follow-up period was 63.3 months (range: 24–120).
| Parameter | Mean | Range |
|---|---|---|
| Output current (mA) | 1.9 | (0.75–3) |
| F (Hz) | 26.9 | (20–30) |
| PW (mic s) | 293.4 | (250–500) |
| OT (s) | 29.6 | (21–30) |
| FT (min) | 2.9 | (0.8–5) |
Group 2 comprised 19 patients with no SBLs: 9 were males (47.3%) and 10 were females (52.6%), with a mean age of 24.8 years (SD 8.8, range: 8 – 38). The mean age at seizure onset was 4.1 years (SD 2.7, range: 1–9), and the mean age at VNS implantation was: 18.3 (SD 7.5, range: 5–30). The mean epilepsy duration was 19.3 years (SD 8.2, range: 2–32). Two patients (10.5%) had prior epilepsy surgery before VNS implantation, including corpus colostomy and functional hemispherectomy. The mean number of AEDs before VNS was three (range: 1–5), and the mean number of AEDs after VNS was 3.2 (range: 2–5). Neuroimaging data were all unremarkable. Eighteen patients (94.7%) had generalized seizures and 1 patient (5.2%) had a focal seizure. Regarding seizure frequency, it was daily in 13 patients (68.4%), weekly in 3 patients (15.7%), and monthly in 3 patients (15.7%). Thirteen patients (68.4%) had other comorbidities, nine (47.3%) had cognitive impairment, and four (21%) had developmental delay.
Regarding VNS settings, Model 102 was used in 1 patient (5.2%), Model 103 was used in 11 patients (57.8%), and Model 106 was used in 6 patients (31.5%). VNS parameters at last follow-up are shown in Table 3.
| Parameter | Mean | Range |
|---|---|---|
| Output current (mA) | 2.1 | (0.75–2.75) |
| F (Hz) | 25.5 | (20–30) |
| PW (mic s) | 312.5 | (250–500) |
| OT (sec) | 28 | (14–30) |
| FT (min) | 3 | (1.1–8) |
Six patients had an average battery usage of 6.5 years (range: 3–9). Five patients (26.3%) reported side effects, including hoarseness, cough, shortness of breath, and wound infection. None discontinued VNS. Regarding the outcome and latest follow-up, 14 patients were responders (73.7%): 4 (28.5%) were R50, 9 were R75 (64.2%), and 1 (7.1%) was SF. Five patients (26.3%) were non-responders. The mean follow-up period was 59.2 months (range: 12–120).
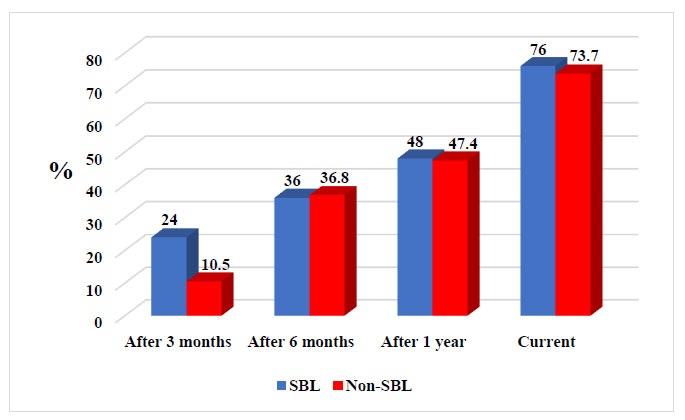
The demographic characteristics of all patients are presented in Table 4. For the two groups with and without SBLs, there was no significant difference regarding sex, mean patient age, mean age at seizure onset, mean age at VNS implantation, mean epilepsy duration, and number of AEDs before and after VNS. There was also no significant difference between groups regarding VNS setting (Table 5). Tables 6, 7 and Fig. (1) show that there was no significant difference between groups regarding the response rate (RR) after 3 months, 6 months, 1 year, and latest follow-up. Unfortunately, some data were missed at a certain time for patients in both groups.
4. DISCUSSION
The treatment of drug-resistant epilepsy is challenging for most epileptologists. Here, we retrospectively analysed patients with medically refractory epilepsy who had received VNS therapy at KFMC, and compared seizure outcomes in patients with and without SBL using a case-control study design.
Our results suggested that over time, patients with SBL received an increased benefit from VNS therapy, similar to non-SBL patients. The data showed that the RR after 3 months was 24%, after 6 months was 36%, and after 1 year was 48%, reaching 76% for those with drug-resistant epilepsy-associated SBL. The mean follow-up period was 63.3 months (range: 24 –120). For non-SBL patients, the RR was 10.5% after 3 months, 36.8% after 6 months, and 47.4% after 1 year, reaching 73.7% over time. The mean follow-up period was 59.2 months (range: 12–120). However, there was no statistically significant difference between the two groups regarding sex, mean patient age, mean age at seizure onset, mean age at VNS implantation, mean epilepsy duration, and mean number of AEDs before and after VNS. In addition, there was no significant difference between groups regarding VNS setting and RR over time (Tables 4, 5, 6, and Fig. 1).
Similar to other studies, the efficacy of VNS was observed in some patients with SBL-associated drug-resistant epilepsy, including those with brain tumors, post-traumatic epilepsy (PTE), and focal cortical dysplasia.
By 2013, there was only one published study evaluating the efficacy of VNS in patients with brain tumour-associated medically intractable epilepsy. The study examined 16 patients, and found that 8 (50%) had an improved outcome (Engel I, II, or III) with an average follow-up of 39.6 months. Outcomes were much better in patients with stable tumors versus more progressing ones. The authors recommended VNS therapy in patients with brain tumor-associated intractable epilepsy, but not in more malignant tumors [41].
Englot and Rolston retrospectively compared seizure outcomes after VNS therapy in patients with PTE to those with nontraumatic epilepsy [42]. The authors found that after VNS therapy, patients with PTE had a greater reduction in seizure frequency compared to non-PTE patients, suggesting that VNS may be considered in patients with medically refractory PTE who are not candidates for surgical intervention [42].
| Group 1 | Group 2 | P-Value | |
|---|---|---|---|
| Sex | Males were 14 (56%), female 11 (44%) | Males were 9(47.3%), Female were 10 (52.6%) | 0.57 |
| Mean age of patients, SD | 25.8 years (SD 11.8) | 24.8 years (SD 8.8) | 0.39 |
| Mean age at seizure onset, SD | 5.3 years (SD 5.6) | 4.1 years (SD 2.7) | 0.19 |
| Mean age at VNS implantation (years), SD | 18.8 (SD 11.9) | 18.3 (SD 7.5) | 0.43 |
| Mean epilepsy duration (years), SD | 20.5 (SD 10) | 19.3 (SD 8.2) | 0.33 |
| Mean AED before VNS | 3.2 | 3 | 0.22 |
| Mean AEDs after VNS | 3.2 | 3.2 | 0.48 |
| Parameter | Group 1 (Mean) | Group 2 (Mean) | P-value |
|---|---|---|---|
| Output current (mA) | 1.9 | 2.1 | 0.17 |
| F (Hz) | 26.9 | 25.5 | 0.18 |
| PW (mic s) | 293.4 | 312.5 | 0.28 |
| OT (s) | 29.6 | 28 | 0.07 |
| FT (min) | 2.9 | 3 | 0.4 |
| Group | 3 Months | 6 Months | 1 Year | Current |
|---|---|---|---|---|
| SBL | 9 NR (36%), 4 R50 (16%), 2R75 (8%), MD 10 (40%). | 6 NR (24%), 4 (16%) R50, 5 R75 (20%), 10 MD (40%) | 6 NR (24%), 8 R75 (32%), 4 R50 (16%), 7MD (28%) | 13 R75 (52%), 6 R50 (24%), 6NR (24%) |
| Non-SBL | 7NR (36.8%), 1R50(5.2%),1 R75 (5.2%), 10 MD (52.6%) | 5 NR (26.3%), 4 R50 (21%), 2 R75 (10.5%), 7 MD (36.8%), 1SF (5.2%) | 4 NR (21.1%), 5 R50 (26.3%), 3 R75 (15.7%),6 MD (31.5%), (5.2%)1SF | 4 R50 (21%), 9 R75 (47.3%), 1 SF (5.2%), 5 NR (26.3%) |
| - | - |
SBL (n = 25) |
Non-SBL (n = 19) |
*P-value | Odds ratio (95% CI) |
|---|---|---|---|---|---|
| After 3 months | Responder | 6 (24%) | 2 (10.5%) | 0.332 | 2.333 (0.356–15.30) |
| Nonresponders | 9 (36%) | 7 (36.8%) | |||
| After 6 months | Responder | 9 (36%) | 7 (36.8%) | 0.619 | 1.071 (0.229–5.018) |
| Nonresponders | 6 (24%) | 5 (26.3%) | |||
| After 1 year | Responder | 12 (48%) | 9 (47.4%) | 0.597 | 0.889 (0.192–4.114) |
| Nonresponders | 6 (24%) | 4 (21.1%) | |||
| Current | Responder | 19 (76%) | 14 (73.7%) | 0.566 | 1.131 (0.287–4.464) |
| Nonresponders | 6 (24%) | 5 (26.3%) |
Furthermore, a meta-analysis study evaluating the efficacy of VNS therapy in patients with drug-resistant epilepsy showed favourable outcomes in the presence of PTE and TS [40].
In our study, three patients underwent surgical intervention, including corpus colostomy and tumour resection, and one patient had intracranial EEG recording. All three patients later developed seizures, raising the question about the impact of failed intracranial epilepsy surgery on VNS effectiveness. In 2011, a study evaluated the impact of failed intracranial epilepsy surgery and other surrogate markers of severe epilepsy on VNS effectiveness in a large cohort with treatment-resistant epilepsy [43]. The authors retrospectively reviewed 376 patients who underwent VNS implantation between 1997 and 2008 and had at least 1 year of follow-up, and found that failed intracranial epilepsy surgery did not affect the response to VNS therapy. Thus, they concluded that VNS should be considered a palliative treatment option for patients with intractable epilepsy, even after failed surgical intervention. Interestingly, in that study, patients with callosotomy did not respond better than those who had resective surgery, and about 50% of patients experienced at least a 50% reduction in seizure frequency. In our study, patients showed a very good RR over time of >70% in both groups.
By 2014, Arcos and Romero [44] evaluated the efficacy of VNS and factors predicting a good response, particularly in the presence of temporal lobe discharge, by video electroencephalogram (video EEG) and magnetic resonance imaging (MRI). The authors noted that the presence of a lesion seen on MRI scan indicates a late and not an early response.
Interestingly, VNS insertion found to render patients with some forms of cortical dysgenesis seizure-free. Actually, this conclusion was conducted from a large study done in Germany, at Epilepsy Centre Bethel. In that study, about 144 patients were evaluated and after insertion, all were examined at regular intervals of 4 weeks for 6–9 months. The results showed that 10 patients remained SF for more than 1 year (6.9%), seizures improved in 89 patients (61.8%), and no changes were observed in 45 patients (31.3%). In fact, different types of malformation of cortical development were observed in 44 patients (30.6%), 5 (1.5%) of whom became SF after insertion. MRI showed focal cortical dysplasia in 34 patients (23.6%), and cerebral dysgeneses such as polymicrogyria, macrogyria, hemimegaloencephaly, and band heterotopia were observed in 6 patients (4.2%). Also, two patients with bilateral posterior parietooccipital polymicrogyria and one with left perisylvian macrogyria became SF. For others, two patients with bilateral perisylvian polymicrogyria and band heterotopia did not improve after VNS, and one patient with hemimegaloencephaly became SF for 4 months [45].
Therefore, despite knowing that resective surgery is the preferred treatment option in well-defined epileptogenic areas [46, 47], VNS has widespread inhibitory effects on the brain, and therefore, has a therapeutic role in patients who are not candidates for surgery [48].
Most studies have reported that <50% of patients with unresected seizure foci and failed resective surgery have a 50% reduction in seizure frequency even with drug trials [49-51], but with VNS, 18% of them have very good outcomes and another 49% shows improvement. Moreover, no significant results have been reported for other surgical options such as subpial trans-sections, callosotomy, and deep brain stimulation in such cases [51-53]. In fact, for widespread dysplasia lesions like (FCD), VNS has been shown to produce responder rates of >50% [52].
A review on VNS therapy [53] noted that in 14 studies, VNS caused a ≥50% reduction in seizure frequency in a median of 50.9% of patients (range: 18.4–67%). In addition, in one randomized controlled trial, VNS implantation resulted in a >50% reduction in seizure frequency in 26–40% of patients within 1 year [54]. These results were almost similar to our findings, as the RR for drug-resistant epilepsy-associated SBL after 1 year was 48%, reaching 76% over time, whereas for non-SBL patients, the RR was 47.4% after 1 year, reaching 73.7% over time. There was no significant difference between both groups (p > 0.05; Tables 4, 5, 6 and Fig. 1).
In addition, we found that 32% of patients with SBL developed complications over time, including hoarseness, cough, shortness of breath, and wound infection, but the majority (16%) had a cough, and about 26.3% of patients in the non-SBL group had complications, with 21% having hoarseness. These results are in accordance with other studies, such as the one conducted by Ghaemi and Elsharkawy [45], who found that 30.6% of patients had complications of which hoarseness was the most common (22.2%), and coughing occurred in 5.6% of patients [45]. However, one of the largest studies performed on VNS patients (247), with a mean follow-up period of 12 years, found that only 8.6% had complications [55], an incidence that is far less than our findings.
The main limitation of this study was the small number of patients; however, we included all patients who underwent VNS implantation in our institute during the past 10 years. A large prospective trial of VNS implantation in patients with and without SBL is necessary to further determine whether patients with or without SBL have the best outcome from VNS insertion; in addition, it would be possible to see which group of SBL patient will show the best response.
Furthermore, people with epilepsy have a 2 to 5-fold higher risk of developing a psychiatric disorder [56] and are more likely to have a poorer quality of life (QOL), in addition to poor self-esteem and a high level of anxiety and depression [57]. Thus, the potential effects of VNS on neuropsychological profiles in patients with SBL-associated intractable epilepsy, and the effect on QOL will be important to consider going forward.
CONCLUSION
The results of this study suggest that VNS is an effective treatment option in patients with intractable epilepsy-associated SBL, who are not candidates for surgical intervention or, despite having surgery, still suffer from seizures; however, more cases and studies are required. Furthermore, VNS showed a cumulative efficacy with time for both entities. No significant difference in response was found in patients with and without SBL.
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
This study has been approved by Institutional Review Board (IRB), King Fahad Medical City, Riyadh, KSA with approval number H-01-R-012.
HUMAN AND ANIMAL RIGHTS
Not applicable.
CONSENT FOR PUBLICATION
Not applicable.
AVAILABILITY OF DATA AND MATERIALS
Not applicable.
FUNDING
None.
CONFLICT OF INTEREST
The authors declare no conflict of interest, financial or otherwise.
ACKNOWLEDGEMENTS
Declared none.


