All published articles of this journal are available on ScienceDirect.
Minimally Invasive Percutaneous Treatment for Osteoid Osteoma of The Spine. A Case Report
Abstract
Osteoid osteomas are benign but painful bone-forming tumors usually involving long bones, with localization at the spine in 10-20% of the cases. The most common symptom is back pain responding to nonsteroidal anti-inflammatory drugs, but in some cases, also radicular pain can be present. For years, surgical excision has been considered the best choice of treatment for cases with unresponsive pain and has been practiced with a high percentage of success but also a high rate of fusion with instrumentation. In the last years, percutaneous radiofrequency ablation has been proposed as a new mini-invasive technique for the treatment of osteoid osteomas.
1. INTRODUCTION
First described by Jaffe in 1953, osteoid osteomas (OO) are benign but painful bone-forming tumors that predominantly occur in the second and third decades of life, with a male preponderance [1-4]. Typically small in size (<15mm), OOs are characterized by a nidus of mineralized immature bone, usually surrounded by osteosclerotic reactive bone and associated with a vascularized stroma. OOs generally affect the long bones; when localized in the spine (10-20% of cases), they usually involve the posterior elements of the vertebrae, such as the lamina, the pedicles, the transverse and spinous processes and the facet joints [2, 4, 5].
The most common presenting symptom is back pain, which typically worsens at night and responds to treatment with nonsteroidal anti-inflammatory drugs (NSAIDs). Indeed, high expression of cyclooxygenase-2 and high level of prostaglandins are found at the nidus and seem to play a key role in inflammation and pain genesis [6, 7]. In some rare cases, OOs can be presented with radicular pain, in particular, when localized near the intervertebral foramen. In these cases, the correct treatment strategy is controversial, and the literature lacks data [8]. Surgical excision used to be considered for decades as the standard treatment in case of unremitting pain, despite conservative treatment, with a high percentage of successes but a high rate of fusion with instrumentation. Spinal fusion has some drawbacks, such as reduction of range of motion and long-term risk of development of adjacent segment disease. With the improvement of the minimally invasive imaging-guided technique over the last years, percutaneous radiofrequency ablation (RFA) has been proposed as a new interventional technique for symptomatic patients with OO [6, 9, 10].
We present a case of a long-time undiagnosed spinal OO causing lumbosciatalgia.
2. CLINICAL CASE
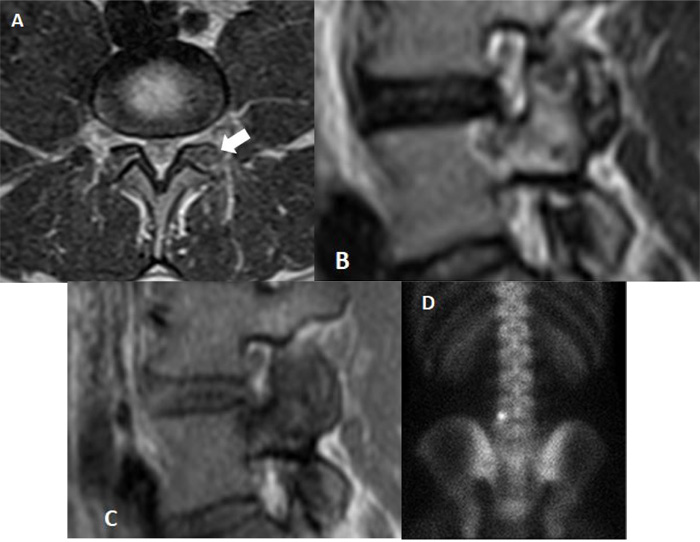
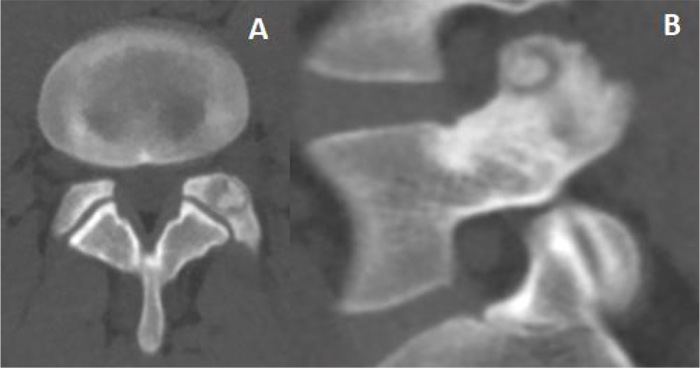
A 30-year-old man was admitted in July 2019 to our clinic. He reported a six year long-lasting history of low back pain, increasing at night and responsive to nonsteroidal anti-inflammatory drugs. Medical and physical treatments have not led to resolution of symptoms, despite the patient increased the dosage and the frequency of the drug administration over the years. On admission, he referred progressive worsening of symptoms, weakness and recent onset of sciatic pain to his left leg. There were no abnormalities in the laboratory studies; no correlation to trauma, sport activity, nor spinal or endocrine disorders was found. At the neurological examination, positive Lasègue sign and mild weakening of the left leg were evident and the physical examination revealed limitation in his low-back range of motion, which was exacerbated by pain. Magnetic resonance imaging (MRI) and thin-slice computed tomography (CT) scan showed an 8 mm large nidus in the left L5 superior articular process, surrounded by intense reactive sclerosis (Figs. 1-2). No alteration of spinal balance or sign of degenerative vertebral disc disease was found. Technetium bone scan localized the lesion, revealing increased uptake of radionuclide in the left posterior side of the L5 vertebra (Fig. 3). Thus, a clinico-radiological diagnosis of osteoid osteoma was made. Due to the tumor localization, we opted for a minimally invasive percutaneous imaging-guided radio-frequency ablation (RFA) to preserve spinal stability and to avoid posterior lumbar fusion. Local anesthesia was administered to control pain during the procedure. The RFA was conducted until reaching a temperature of 90°, which is considered the standard to achieve a complete ablatio. The patient was observed for 2 hours, then discharged on the same day, with a 10-day clinical check-up. The day after the procedure, a transient exacerbation of sciatic pain in the left leg occurred, responding to anti-inflammatory drugs. At 6-month follow-up, the patient did not report significant discomfort and was asymptomatic.
3. DISCUSSION
OOs are relatively rare bone tumors (almost 10% of all benign bone tumors), usually involving the long bones; vertebral localization is not so common (up to 20% of cases) with a prevalence in the posterior lumbar column [2, 11].
Due to its small size, nidus is not easily detected by plain radiographs. Magnetic resonance imaging is helpful for the differential diagnosis [12-16]. The T2-weighted magnetic resonance images can identify the nidus and the perinidal bone marrow edema, which could be the only finding for an osteoid osteoma. The main contribution of MRI is to discover the possible spinal cord or nerve roots involvement and soft tissue inflammation [12, 14, 17-19]. Computed tomography is helpful to clarify the nature of the lesion, it well defines the anatomical features of the nidus and the extent of perinidal sclerosis. Thin-layer CT is crucial to pinpoint the nidus for preoperative planning.
OOs are well individuated by Technetium-99m bone scintigraphy due to their hypervascular composition with evidence of intense radionuclide uptake [20, 21].
Due to its benign nature, with no potential for malignant degeneration, OOs have a good prognosis. Spontaneous regression has been reported in the literature, occurring at 33 months [10], therefore, some authors consider conservative management with NSAIDs.
Long-time administration of NSAIDs should be avoided for their potential negative effects. The goal of treatment remains the tumor removal.
Owing to their classical posterior localization, the management of spinal OOs is a challenge.
Surgical “en bloc” resection of the nidus and drilling of the surrounding bone sclerosis has been considered the best choice for years, with a success rate of 88 to 100%. Although it provides the benefit of histopathological diagnosis, surgery also increases the risk of transitory or permanent morbidity and may lead to some complications.
In our case, the OO involved the superior articular process and the left lamina of L5. The excision of the tumor would have required the removal of the facet joint and partial laminectomy could have resulted in spinal instability with the necessity of a posterior lumbar fusion and with important consequences on spinal flexibility and range of motion. This is in accordance with literature that reports a fusion rate of 20-50% after surgical procedures [3, 17, 22-24]. Minimally invasive percutaneous image-guided techniques are preferred in young patients without previous neurological compromise and instability.
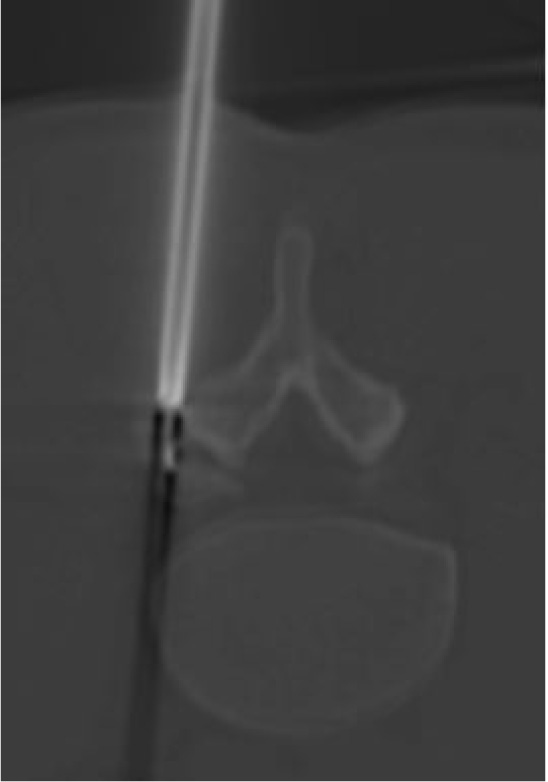
Radiofrequency ablation CT-guided is the treatment of choice for small OOs, with a reported success rate of up to 90%, and a complication rate of less than 2% [6, 25]. Compared with the traditional surgical approach, RFA is more cost-effective, reduces the risk of blood loss and postoperative pain and does not generally require hospitalization. It can be performed in local anesthesia; rarely general or epidural anesthesia is required to avoid inadvertent movements of the patient. Indeed, the main drawback of this technique is the possibility of injury of surrounding structures, in particular for the spinal roots, that can range from nerve root irritation to transient or permanent neurological deficit. For this reason, the patient must be informed of the potential risks and benefits of the procedure. Recurrences (rate near to 5%), often within 6 months of treatment, due to incomplete ablation of the nidus, are described [4]. Therefore, it is important to maintain a temperature of 90° for 5-6 minutes to guarantee a satisfactory ablation of the nidus [25]. CT is helpful to recognize and center the target, providing quick acquisition, thin slices, high-image resolution and the possibility of 3D reconstruction for preoperative planning [12, 26].
CONCLUSION
Spinal OO is a rare entity and its characteristic pain is often misinterpreted as radicular. No evidence of disc herniation or nerve root compression at MRI can guide to diagnosis.
Surgery is not suitable for young patients and should be reserved only in the case of preoperative neurological compromise or spinal deformity, such as in the case of OO-related scoliosis [27].
Imaging-guided RFA is currently considered the treatment of choice but has a relative risk of thermal injury to the nerve roots, so proper preoperative planning has to be made and adequate informed consent must be obtained from the patient.
ABBREVIATION
| OO | = Osteoid osteoma |
| NSAIDs | = Nonsteroidal anti-inflammatory drugs |
| RFA | = Radiofrequency ablation |
| MRI | = Magnetic resonance imaging |
| CT | = Computed tomography. |
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
Not applicable.
HUMAN AND ANIMAL RIGHTS
No animals/humans were used in the studies that is the the basis of this research.
CONSENT FOR PUBLICATION
Not applicable.
STANDARDS OF REPORTING
CARE guidelines and methodology have been followed.
FUNDING
None declared.
CONFLICT OF INTEREST
The authors declares no conflict of interest, financial or otherwise.
ACKNOWLEDGEMENTS
Declared none.


