All published articles of this journal are available on ScienceDirect.
Oral Cladribine is Effective in the Treatment of Tumefactive Demyelination in Relapsing Remitting Multiple Sclerosis – A Case Report
Abstract
This case report describes a 32-year old Emirati patient with tumefactive multiple sclerosis (TMS) treated with a short course of cladribine tablets. The patient presented initially with acute onset of progressive gait unsteadiness, lower limb weakness, associated with numbness of four days duration. Neurological examination of right arms and both legs weakness with sensory level at T10. Contrast-enhanced magnetic resonance imaging (MRI) of the brain showed a large tumefactive demyelination with multiple demyelinating lesions in the brain, consistent with a diagnosis of multiple sclerosis. MRI of the spine cervical and dorsal revealed multiple enhancing lesions as well. Cerebrospinal fluid oligoclonal bands were positive, and IgG index was high. Magnetic resonance spectroscopy showed elevation of lactate on short echo time (TE) and elevation of choline on long TE. The patient was treated with pulse steroid followed by oral cladribine as immune reconstitution therapy in the acute phase. The patient showed significant improvement clinically and radiologically to the treatment. The patient was followed up for 2 years and showed excellent resolution of the tumefactive lesion with no new lesions. Immune reconstitution therapy can be an option for treatment of tumefactive demyelination in multiple sclerosis in the acute setting. To our knowledge, there are no reports of the use of immune reconstitution therapies for the treatment of tumefactive lesions in multiple sclerosis.
1. INTRODUCTION
Tumefactive demyelinating lesions (TDL) or tumefactive multiple sclerosis (TMS) though used interchangeably, are not the same. Tumefactive demyelination can be caused by a variety of causes including Sjogren’s disease, lupus erythematosus, neuromyelitis optica, human immunodeficiency virus (HIV), acute disseminated encephalomyelitis, multiple sclerosis, and drugs. Tumefactive lesions in multiple sclerosis are defined as large (>2 cm) tumor-like demyelination in the central nervous system with surrounding edema, mass effects and ring enhancement, or 0.5 to 2 cm demyelination with possible mass effect and having a characteristic radiographic appearance [1, 2]. Prevalence of tumefactive demyelination has not been formally evaluated and is estimated to be 1-3/1000 cases of multiple sclerosis with an annual incidence of 0.3/100,000 [3]. TDL may be the first initial presentation in multiple sclerosis (53-78%) or may occur in patients with pre-existing multiple sclerosis [4, 5]. Diagnosis of tumefactive demyelination is challenging and depends on the clinical and typical radiological characteristics, and may need biopsy in certain cases for confirmation. There are no standard guidelines for management of TDL. Many therapies, including Intravenous corticosteroids, plasmapheresis, rituximab, cyclophosphamide and natalizumab have been tried previously in the acute setting with variable results. Immune reconstitution therapies (IRTs) have never been used in TDL.
We herein report a patient with tumefactive demyelination in relapsing remitting multiple sclerosis who successfully responded clinically and radiologically to oral cladribine.
2. CASE REPORT
A 32 year old gentleman presented with a four day history of progressive weakness and numbness in the right arm and both legs, with greater weakness in the right leg. The weakness progressed to a point where he was unable to stand or walk by himself and had to come in a wheelchair to the hospital. There was no preceding fever or associated trauma, neck pain or back pain. There was no double vision or blurring of vision. In the past, he had suffered from many episodes of imbalance, dizziness, urinary urgency and incontinence; each of these lasted for a few days to a week and abated gradually. He had not consulted anyone for these symptoms or had no investigations done. He did not have any history of skin rash, joint swelling or orogenital ulcers. There was no history of any substance abuse or alcoholism. There was also no family history of neurological problems.
Examination revealed normal higher mental functions. Lhermitte’s sign was negative. Cranial nerve examination was unremarkable except for right supranuclear facial weakness. Right upper limb power was 3/5. Power in the right leg was 3/5 and left leg was 4/5, with exaggerated deep tendon reflexes and bilateral extensor planters. Sensory level for touch and pinprick was D10. There were no cerebellar signs. Expanded Disability Status Scale (EDSS) was 7.0. Laboratory tests, including complete blood count, liver function, renal function, erythrocyte sedimentation rate (ESR), and anti-nuclear antibody (ANA) profile were normal. Cerebrospinal fluid (CSF) study showed normal protein and glucose while cell count was normal. Oligoclonal bands were positive. Visual evoked potential (VEP) was normal in both eyes. Magnetic resonance imaging (MRI) of the brain with and without contrast showed multiple bilateral white matter with T2/FLAIR hyperintense lesions involving periventricular and juxta-cortical regions as well as the cerebellum and the brainstem (Fig. 1A, B and C). Some of the lesions showed low signal in T1 and subtle rim enhancement on post contrast images. In addition, enhancing lesions were seen in the occipital region and the right cerebellum, consistent with active lesions of multiple sclerosis. In addition, there was an oval-shaped lesion in the left frontal centrum semi ovale, with hyperintense signal in T2 and hypointense in FLAIR surrounded by perifocal oedema with minimal mass effect. The lesion demonstrated minimal faint rim enhancement and measured 2 cm x 2.5 cm x 4 cm (transverse x antero-posterior x cranio-caudal, respectively) and did not show any restricted diffusion. On MRI spectroscopy, lesion showed elevation of lactate on short echo time (TE) and elevation of choline on long TE. N-acetyl aspartate showed a reduction on both TE, supporting a diagnosis of a tumefactive demyelinating lesion.
Spine MRI with contrast revealed multiple focal T2 high signal lesions in cervical spine with some of the lesions demonstrating enhancement in post contrast images (Fig. 1D). One lesion seen in the thoracic spine between T8/9 (Fig. 1E) demonstrated post contrast enhancement and was probably a symptomatic lesion. The rest of the thoracic and lumber spine was normal.
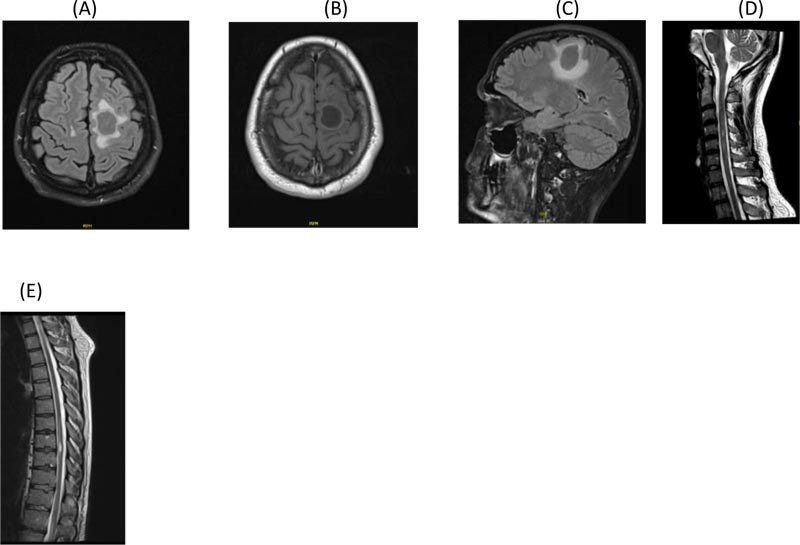
A. Axial Flair brain MRI showing left parietal tumefactive demyelination
B. Post contrast axial T1 brain MRI showing ring enhancement of the left parietal tumefactive demyelination
C. Sagittal Flair brain MRI showing parietal tumefactive lesion with significant surrounding edema before treatment
D. Sagittal T2 cervical post contrast MRI showing enhancing plaque opposite C4 vertebra
E. Dorsal Spine T2 post contrast MRI showing D7/8 enhancing lesion before treatment
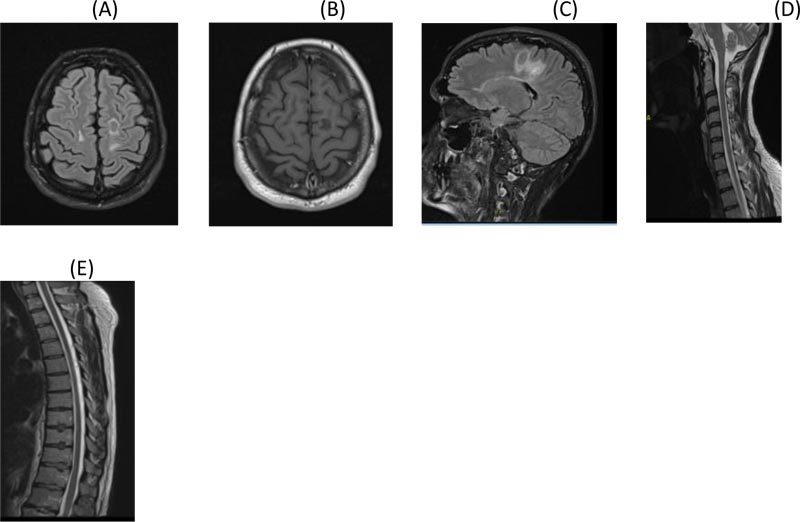
A. Axial Flair brain MRI
B. Post Contrast axial T1 brain MRI showing significant resolution of lesion with no enhancement
C. Sagittal Flair brain MRI showing significant reduction in size and edema of the tumefactive lesion
D. Sagittal T2 cervical post contrast MRI
E. Dorsal Spine T2 post contrast MRI
Diagnosis of tumefactive demyelination in highly active multiple sclerosis was established on the basis of patient’s history of recurrent episodes of neurological deficit, positive CSF oligoclonal bands and typical MRI findings. For acute relapse, he was given a 5-day course of intravenous methylprednisolone 1 gm, after which he started improving gradually. As he was a case of highly active multiple sclerosis, the options of starting first line therapy such as Natalizumab, Alemtuzumab, Ocrelizumab and Cladribine were discussed with the patient and his family. The MENACTRIMS guidelines offer these options for highly active multiple sclerosis as first line and are followed in this region [6]. As there are no specific guidelines for the treatment of tumefactive demyelination in MS, all the above options were considered with their risk benefit ratio. The patient and his family opted for oral cladribine with its ease of administration protocol and safety profile.
On day 7 of his attack, the patient started taking cladribine 10 mg, 7 tablets (over 5 days) in Month 1 and 7 tablets in Month 2 orally. The dose was calculated as per his body weight. When reviewed in Month 3, the patient was much better. He was walking with a mild limp in the right leg and his sensory exam was normal. EDSS was 2.5. MRI of brain and spine were repeated in Month 3 and showed a significant resolution of the tumefactive lesion and the spine lesions as well (Fig. 2A, B, C, D and E). At 6 months follow-up, he had recovered completely. One year post relapse, he had another MRI of the brain and spine which showed further resolution of the tumefactive lesion and spine lesions (Fig. 3A, B, C and D). Patient had no symptoms and his EDSS was 1.0. Same course of cladribine was repeated after one year. He was reviewed after 2 years of the first course and follow-up MRI of brain and spine continued to show further resolution of the tumefactive lesion, with no new T2 or enhancing lesions (Fig. 4A and B). Absolute lymphocyte counts dropped to Grade 1 lymphopenia after 3 months which recovered to normal levels thereafter, after each course.
3. DISCUSSION
There are no standard guidelines for the treatment of tumefactive demyelination in multiple sclerosis. High dose methylprednisolone has been used as the first line treatment and has shown mixed results [4]. Plasma exchange appears to be a reasonable second line therapy in those with poor response to corticosteroids [7]. However, sometimes prolonged therapy for as many as 21 cycles may be required for a favorable response [8]. Rituximab has been used successfully in patients with refractory symptoms despite treatment with steroids, immunoglobulins and plasma exchange [9]. Cyclophosphamide and intravenous immunoglobulin appear to be effective in treating TMS in children, but the effectiveness in adults is limited [10, 11]. Evidence on effective disease modifying therapy in TMS is limited. Although previously interferon-β and glatiramer acetate were commonly used in TMS, there are no data available to show that the efficacy of these first line treatments is different in patients with TD lesions compared with typical relapsing remitting MS (RRMS) or whether one of these is superior to the other [4]. Fingolimod and natalizumab have been associated with an increase in the size of TD lesions [12, 13].
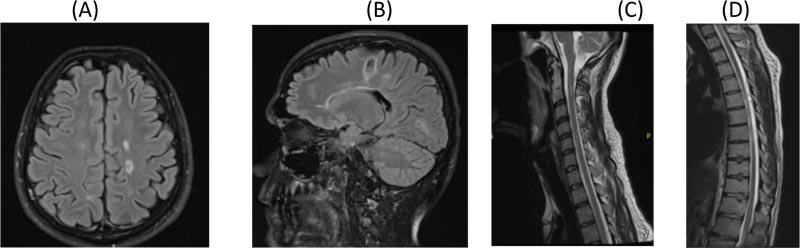
A. Axial Flair brain MRI
B. Sagittal Flair brain MRI showing further resolution of tumefactive lesion
C. Sagittal T2 cervical post contrast MRI showing further resolution of C4 lesion
D. Dorsal Spine T2 post contrast MRI showing further resolution of D7/8 lesion
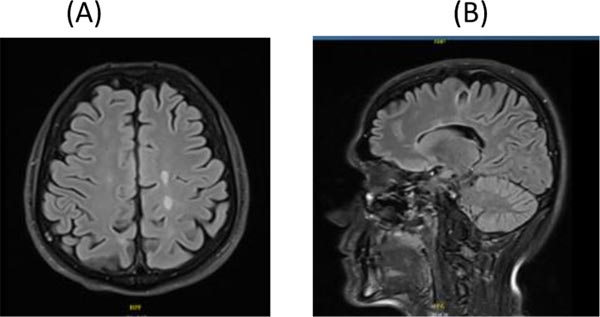
A. Axial Flair brain MRI
B. Sagittal Flair brain MRI showing resolution of the tumefactive lesion
It is imperative to start DMTs early once the diagnosis of RRMS is established in order to reduce inflammation and secondary axonal loss in the CNS. Multiple studies have shown that early treatment might decrease the long-term accumulation of disability in patients with MS [14, 15]. The choice of initial therapy will depend on the extent of disease activity. Studies have shown that high disease activity (HDA) early in the course of the disease is predictive of future disability accumulation [16-18]. Both concepts of in- duction i.e. using high efficacy DMTs early in the disease course vs escalation i.e. starting with low efficacy DMTs and escalating if response is suboptimal, have been proposed for managing patients with RRMS. Unfortunately, there is no current consensus on defining highly active disease in RRMS. The following clinical and radiological features should be taken into consideration when determining if a patient has a highly active disease:
- Relapse frequency in the previous year (≥2 relapses).
- Relapse severity (pyramidal/cerebellar systems involvement).
- Incomplete recovery from relapses.
- High T2 lesion load on MRI (≥10 lesions), especially with spinal or infratentorial lesions.
- Multiple Gadolinium enhancing lesions.
Our patient fulfills the criteria for highly active disease with tumefactive demyelination. After having a detailed discussion with the family, we agreed on oral cladribine to be used in the acute phase as IRT.
IRTs have never been used to treat TD lesions acutely in patients with multiple sclerosis. An IRT, by definition, is given at short intermittent courses and not continuously [13]. IRT modalities were shown to induce long-term remission of MS that, in some cases, is close to the definition of a “cure”. Other IRTs that are used in MS include alemtuzumab and hematopoietic stem cell transplantation (HSCT) [14].
Cladribine, a structural analogue of deoxyadenosine, is well-tolerated with prolonged immune reconstitution properties. In the cells, cladribine is phosphorylated by deoxycytidine kinase to cladribine phosphate, which accumulates intracellularly to interfere with DNA synthesis and repair, ultimately resulting in DNA strand breaks and cell death [20]. The conversion of cladribine to its active phosphate form is particularly efficiently achieved in lymphocytes. Thus cladribine selectively reduces circulating B and T lymphocytes; this reduction is sustained over time and the counts return to a normal level over one year of treatment [20]. Cladribine has also been shown to increase the levels of anti-inflammatory cytokines such as interleukin (IL)-4, IL-5 and IL-10 and penetrate the blood-brain barrier [21, 22]. We postulate that because of these immune-suppressive effects, cladribine rapidly reduces the size of tumefactive demyelinating lesions in multiple sclerosis. A short course of cladribine treatment thus provides long-lasting and effective suppression of disease activity.
Our patient was a case of highly active MS with tumefactive demyelination who after receiving steroid along with oral cladribine in the acute phase showed significant and early response clinically and radiologically.
The strength of this case report is that it uses the novel concept of immune reconstitution therapy for acute treatment of tumefactive lesions in multiple sclerosis. The drawbacks focus on only one drug, namely cladribine.
CONCLUSION
This case showed a good clinical and radiological effect of cladribine on tumefactive demyelinating lesions in multiple sclerosis. Immune reconstitution therapy can be an option for the treatment of tumefactive demyelination in Multiple sclerosis in the acute phase. This case report may suggest the potential to study further the effect of cladribine therapy on patients with TDL in the acute setting.
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
The study has been approved by DSREC (Dubai Scientific Research Ethics Committee). Reference for approval: part of the U.A.E MS Registry: DSREC-09/2015_06.
HUMAN AND ANIMAL RIGHTS
Not applicable.
CONSENT FOR PUBLICATION
Informed consent was obtained obtained from the participant.
STANDARD OF REPORTING
CARE Guideline and methodology were followed.
AVAILABILITY OF DATA AND MATERIALS
Not applicable.
FUNDING
The development of this publication was financially supported by Merck Serono Middle East FZ LTD an affiliate of Merck KGaA, Darmstadt, Germany through an independent medical writing grant.
CONFLICT OF INTEREST
The authors declare no conflict of interest, financial or otherwise.
ACKNOWLEDGEMENTS
The contributions of the authors are as follows: Dr. Jihad is the main author and treating physician of the patient, Dr. Mona and Dr. Pournamy addressed revisions to the manuscript, Dr. Nouf provided the MRI images, and Dr. Abubaker supervised the processes in the clinic. Dr. Rupali Bahri from Medcytes, Dubai provided medical writing services for development of the manuscript.


