All published articles of this journal are available on ScienceDirect.
Botulinum Toxin for the Treatment of Focal Task-Specific Hand Dystonias: Systematic Review and Meta-Analysis
Abstract
Objective:
Botulinum Toxin (BTX) has become a widely used treatment in several dystonic conditions, but the evidence for its efficacy has largely come from open trials and expert opinion. This systematic review examined the efficacy and safety of BTX in the treatment of Focal Hand Dystonia (FHD) in Randomized Controlled Trials (RCTs).
Methods:
We searched Ovid MEDLINE, EMBASE, Cochrane Database of Systematic Reviews (CDSR), Database of Abstracts of Reviews of Effects (DARE), Cochrane Central Register of Controlled Trials (CENTRAL), CINAHL, SPORT Discus, SCOPUS, Web of Science, PEDro, ClinicalTrials.gov, and the International Clinical Trials Registry Platform (ICTRP) for randomized, placebo-controlled trials on the use of BTX for FHD.
Results:
Of 1,116 publications retrieved from the databases searched, three publications were included. The evidence identified pertains to focal task-specific hand dystonias. Sixty-nine participants were involved in the three RCTs with a mean duration of symptoms of 7.5 years. Participants were assessed using a combination of self-reported and functional performance outcome measures following injections of BTX subtype A (BTX-A) or placebo. The Oxford Quality Scale was used to assess the included studies, and the three studies each scored 3/5 or above. The included studies reported no adverse events with BTX-A use, other than muscle weakness and pain at the injection sites.
Conclusion:
The number of participants included in these three trials is too small to draw dependable conclusions about the efficacy and safety of BTX-A for FHD. There is currently not enough evidence to recommend the routine use of BTX-A for FHD.
1. INTRODUCTION
Dystonia is a disabling neurological syndrome that can produce involuntary, sustained or spasmodic movements and abnormal postures [1, 2]. The pathophysiology of dystonia is complex and it seems likely that dysfunction in the basal ganglia or linked central pathways leads to abnormal motor output from the central nervous system. Complex movements, such as writing or playing the piano, can be particularly affected. Focal Hand Dystonia (FHD) manifests as a loss of motor control while performing a specific skilled repetitive hand action (e.g. musician’s dystonia, writer's cramp). Such movements require exquisite motor control with excitatory and inhibitory signals controlling appropriate muscle contractions whilst simultaneously relaxing surrounding muscles. This “surround inhibition” is abnormal in patients with dystonia with both agonist and antagonist muscles in a limb segment co-contracting [3].
Botulinum Toxin (BTX) may help patients with dystonia by weakening specific overactive muscles, but there is often a fine balance between making a muscle too weak and not weakening it enough. While the use of BTX in other types of dystonia such as cervical dystonia is supported by a larger evidence base with respect to efficacy, evidence reviews for the use of BTX as a treatment for FHD are limited [4, 5]. An up-to-date knowledge synthesis on the use of BTX for FHD has the potential to impact clinical decision making and guideline development.
The objective of this systematic review is to summarize the current literature relating to the efficacy and safety of BTX in the treatment of FHD as assessed in Randomized Controlled Trials (RCTs).
2. METHODS
This systematic review followed the recommendations outlined in the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement [6].
2.1. Criteria for Study Inclusion
In order to be eligible for inclusion, clinical trials needed to be randomized, double-blind, and placebo-controlled. The treatment group should have received BTX and the control group an inactive sham or placebo. Participants needed to have a clinical diagnosis of FHD and should not have undergone specific additional treatment for their dystonia as part of the trial.
2.2. Searching
A systematic literature search was conducted by a health sciences librarian (MCT) using the following databases:
- Ovid MEDLINE® Epub Ahead of Print, In-Process & Other Non-Indexed Citations, Daily and 1946 to present
- Ovid Embase 1974 to present
- Wiley Cochrane Library: Cochrane Database of Systematic Reviews (CDSR)
- Wiley Cochrane Library: Database of Abstracts of Reviews of Effects (DARE)
- Wiley Cochrane Library: Cochrane Central Register of Controlled Trials (CENTRAL)
- EBSCOHost CINAHL Plus with Full Text 1937 to present
- EBSCOHost SPORT Discus with Full Text
- SCOPUS
- Web of Science Core Collection
- PEDro Physiotherapy Evidence Database
An auxiliary search was also conducted in the following clinical trial registries:
- ClinicalTrials.gov
- World Health Organization International Clinical Trials Registry Platform (ICTRP)
All searches were conducted between September 28 and 29, 2017. The MEDLINE search strategy was developed by MCT and then was peer-reviewed by a librarian expert searcher at the University of Alberta. The MEDLINE search strategy was then translated to search strategies suitable for the other databases.
The search strategy included a combination of controlled vocabulary terms (e.g., Medical Subject Headings (MeSH) and keywords representing the concepts of “hand dystonia” and “botulinum toxin”. The full search strategy for all the databases searched is available in Appendix A of this publication. A search filter was applied for RCTs and to reduce retrieval of animal studies, when feasible. No language, date, or other limits were applied in the electronic database searches. Additional references were identified in the following ways: by hand-searching reference lists of all included studies as well as pertinent review articles; by forward citation searching in Scopus to find studies citing any of the included articles; and by searching conference proceedings for abstracts, posters and presentations on FHD and BTX treatment. Results were exported to the RefWorks citation management system [7] and subsequently imported into Covidence [8], which was used for deduplication, as well as title/abstract, and full-text screening. Three authors (DA, AD, and HA) duplicated the title/abstract and full-text screening; while data extraction was performed in duplicate by CG, DG, and HA. Disagreements would have been resolved by SS.
2.3. Study Quality Assessment
For the assessment of risk of bias, all eligible trials were assessed using a three-item, five-point scale, the Oxford Quality Scale [9], that considers randomization, blinding, study withdrawals and dropouts, and a consensus score was agreed upon. Each study was assessed in duplicate (CG, DG and HA). Disagreements were resolved by SS.
2.4. Outcomes
Our primary outcome of interest was hand function as measured by any validated hand function assessment at two weeks to two months post-treatment.
Our secondary outcomes included:
- Any validated assessment of hand function at two to six months post-treatment
- Reduction in secondary symptoms (e.g. pain, tremor)
- Any assessment of quality of life
| First Author and Year | Type of Study | Number of Participants | Participant Sex | Mean age (range) in years | Diagnoses of Participants | Mean Duration of Symptoms (range) in Years |
|---|---|---|---|---|---|---|
| Tsui (1993) [10] | Cross-over trial | 20 | 7F 13M |
41 (28–58) | 20 WC | 5.2 (2–14) |
| Cole (1995) [11] | Cross-over trial | 10 | 7F 3M |
49 (36–66) | 6 WC 2 SC 2 MC |
8.5 |
| Kruisdijk (2007) [12] | Parallel-group trial | BTX-A group: 20 |
10F 10M |
BTX-A Group: 48 |
39 WC | BTX-A Group: 7.4 |
| Placebo: 19 | 7F 12M |
Placebo: 46 | Placebo: 9.1 |
- Number of participants with any adverse events
- Number of participants with any serious adverse events
- Number of participants withdrawing due to adverse events
2.5. Meta-Analysis
For our meta-analysis, we computed a Relative Risk (RR) with a 95% Confidence Interval (CI). Between study heterogeneity was assessed with the I-squared statistic and a random effects model was used for our meta-analysis because of the heterogeneity.
3. RESULTS
3.1. Search Results
Our searches yielded 1,116 publications from the databases searched with 674 publications remaining after deduplication. No additional references were identified from other sources. Applying the eligibility criteria to the titles and abstracts screened resulted in 24 articles left for full-text evaluation. Ultimately, three studies [10-12] met our inclusion criteria. Fig. (1) shows the PRISMA flow diagram. The publications excluded at the full-text stage and the reasons for exclusion are presented in Appendix B. As regards the included studies, two used a cross-over design and one used a parallel-group design. A total of 69 participants were involved in the three trials which included 31 women and 38 men. The evidence identified pertains to focal task-specific hand dystonias. Two of the studies were limited to participants who had been diagnosed with Writer’s Cramp (WC), and overall the majority (94%) of the participants had WC. The mean duration of symptoms in the participants was 7.5 years. Table 1 details the study designs and demographic characteristics of participants.
3.2. Study Quality Assessment
One study was given an Oxford Quality Scale score of 5/5 and two studies a score of 3/5. Tsui et al. [10] was given an overall quality score of 3/5 (1 point for randomization, 1 for blinding, 1 for description of dropouts). Similarly, Cole et al. [11] scored 3/5 (1 for randomization, 1 for blinding, and 1 for description of dropouts) and Kruisdijk et al. [12] achieved a maximum score of 5/5.
3.3. Efficacy
Table 2 shows the muscles targeted, BTX dosages used, the outcome assessments employed, and the main results of the included studies. Identification of muscles to be injected was undertaken clinically in all the three trials and supplemented by Electromyographic (EMG) assessment in two [10, 11] of the three studies. The study authors used intramuscular injections of BTX-A or equal volumes of saline in all the trials. Doses of BTX-A varied between the participants depending on the selection and number of muscles. Generally, participants were assessed prior to the intervention, and at two weeks to one-month post-intervention, or when any treatment effect had worn off.
The authors of the studies used a combination of self-report by the participants and functional performance measurements. Different self-report questions were used. Tsui et al. [10] asked participants to report whether they noticed any changes in writing, which was scored as follows: -1 = worse; 0 = no change; 1 = improvement; and 2 = dramatic improvement. Cole et al. [11] requested participants to complete a questionnaire about the amount of improvement of their muscles at the time of each assessment, using a categorical scale (major, moderate, minimal, or none), and a Visual Analog Scale (VAS). The VAS ranged from 0 (no improvement) to 10 cm (return to normal). Kruisdijk et al. [12] used the participants’ answers to the following question as their primary outcome measure: “Considering all advantages and disadvantages of this treatment, is the improvement such that you wish to continue this treatment or not?” Functional performance measures included videotape analysis of writing samples, speed and accuracy of pen control, Gibson’s maze assessment [13] (a spiral maze in which the participant is asked to trace the path in the maze from the center and work spirally outward with the pen as quickly as possible), Symptom Severity Scale (SSS) [14], Functional Status Scale (FSS) [14], and Writer’s Cramp Rating Scale (WCRS) [15]. The specific scales used for functional performance assessments varied considerably between the three trials.
Tsui et al. [10] undertook a randomized controlled cross-over trial of 20 participants with WC. They used three functional performance tests and the authors reported statistically significant improvement in the BTX-A treated participants in pen speed and accuracy, in three out of four movement directions at two weeks, using a digitized pad. The study authors did not specify how the statistical analysis was performed and none of the placebo group results were reported in the paper. The authors did not respond to our email enquiry regarding this matter. Performance in the Gibson’s maze task improved significantly in 9/20 participants (45%) for speed, and 7/20 participants (35%) for accuracy with BTX-A. Writing speed improved in 7/20 participants (35%) and the writing was more legible in 4/20 participants (20%), but no statistical testing was reported.
| First Author / Year | Hand Muscles Affected | Intervention/Type of Dose of BTX-A | Type of Assessments/Outcome Measures | Main Results |
|---|---|---|---|---|
| Tsui (1993) [10] | FDS, FCR, FCU, FPL, EDC, ECU, PT, PQ |
BTX-A injection – dose range 25–50 MU |
1. Self-report assessment of participant writing on a four-point scale 2. Speed and accuracy of pen control 3. Gibson’s maze 4. Copying a standard passage (readability scoring by “blind” physician) |
Self-reported results were modest: 4/20 had definite improvement, 2/20 had slight improvement. 8/12 participants described pain relief after BTX-A. For speed, significant mean change in velocity from baseline to 2 weeks for BTX-A was recorded for horizontal movement (0.17 cm/sec, p < 0.05); for vertical movement (0.9 cm/sec, p < 0.01); and for descending movement (0.39 cm/sec, p < 0.05). Mean time to complete Gibson’s maze significantly improved by 3.23 sec (p < 0.01) at 2 weeks, and by 3.63 sec (p < 0.01) at 6 weeks for participants on BTX-A. |
| Cole (1995) [11] | FCR, APL, EPL, FDS, FPL, FCU, FDP, EDC | BTX-A injection – dose range 5–30 MU |
1. Self-report response about improvement and weakness 2. Functional performance testing using: a. MRC scales for muscle power b. Timing of writing sample c. Counting errors of writing “off-the-line” for WC d. Counting the number of errors for a standard transcription for SC e. MC participants played a standard piece of music which was recorded on videotape and assessed by five professors of music |
4/10 participants had major improvement, 5/10 had moderate improvement and 1/10 had no improvement to BTX-A. From MRC testing, 7/10 participants were weaker with BTX-A, 3/10 had no difference in strength after BTX-A, 2/10 had weakness after placebo. 4/6 participants with WC wrote faster after BTX-A. 2/2 participants with SC did not show any improvement in BTX-A compared to placebo. 2/2 participants with MC improved on BTX-A compared to placebo. |
| Kruisdijk (2007) [12] | FPL, FDP, EIP | BTX-A injection – 178 MU (range 30–240) Placebo – volume equal to 224 MU (20–280) |
1. Self-response to the question about benefit of treatment 2. Secondary outcome measures included: a. Visual analogue scale (VAS) for handwriting b. Symptom severity scale (SSS) c. Functional status scale (FSS) d. Writer’s cramp rating scale (WCRS) e. Writing speed |
Primary outcome – In the BTX-A group, 14/20 participants wished to continue treatment versus 6/19 participants in the placebo group. Secondary outcome – The mean difference comparing placebo to BTX-A from baseline to 8 weeks of the objective measures were: VAS: 1.32 cm (95% CI, 0.33 to 2.3, p = 0.01) in favor of BTX-A. SSS: -2.44 points (95% CI, -4.52 to -0.37, p = 0.02) in favor of BTX-A. FSS: 2.07 points (95% CI, -0.4 to 4.54, p = 0.1). WCRS: -1.51 points (95% CI, -2.55 to -0.47, p < 0.01) in favor of BTX-A. Writing speed: 1.14 lines (95% CI, 0.07 to 2.20, p = 0.04) in favor of BTX-A. |
Of interest, participants who had abnormal wrist posture in association with their dystonia were reported to have a significantly stronger positive response to the BTX-A therapy. Eleven out of 13 participants (85%) with wrist-joint deviation improved on pen control versus 1/7 participants (14%) without deviation; 8/13 participants (62%) with wrist deviation improved on Gibson’s maze versus 1/7 participants (14%) without deviation; and 7/13 participants (54%) versus 0/7 improved on self-report with regards to writing. A chi-square analysis was reported comparing these two groups and the difference was found to be significant (p<0.001).
Eight out of 12 participants (67%) with pain noted improvement in their symptoms following BTX-A injection but no statistical analysis was reported. The self-reported assessment of writing found 6/20 participants (30%) reporting improvement in writing, 13/20 participants (65%) with no change, and 1/20 participants (5%) transiently worse with BTX-A. The study authors reported that there were no changes in self-reported writing of participants during the placebo phase.
Cole et al. [11] performed a randomized cross-over trial of 10 participants comparing BTX-A with saline injections at two to four weeks post-injection. These were highly selected participants in that they came from the study authors’ existing clinic population and were patients “who stated that they had a definite subjective response to open-label treatment with BTX-A”, and, furthermore, whose dose of BTX-A had been optimized already. This study, therefore, is a complete enriched enrolment trial [16]. The washout period varied from two weeks to four months depending on whether the “patient and physician determined that the [prior] injection might have had some effect”. None of the results from any of the outcomes of the trial (either subjective feeling of ‘improvement’ by the patient or the objective measures of changes in muscle strength, writing speed, transcription speed, physician rating of motor performance of all participants and rating of musical performances by professors of music who were blinded to the treatment groups) were subjected to statistical analysis and the results were therefore purely descriptive. Manual muscle testing using the Medical Research Council (MRC) scale [17] found that 7/10 participants (70%) were weaker when injected with BTX-A compared to placebo and 2/10 participants (20%) were weaker with placebo than with BTX-A. For participants with WC, the time required to write a standard paragraph was recorded, and errors of writing “off-the-line” were counted and used for objective assessment. Four out of six participants (67%) with WC wrote faster after BTX-A, and 2/3 participants (67%) who wrote “off the line” improved after BTX-A injection. One participant who was only able to print after placebo could write a standard paragraph in longhand after BTX-A. Two stenographers did not reduce their transcription error rate after BTX-A. Two musicians judged by a panel of professors of music were thought to give better performances after BTX-A injections. The study authors found that 9/10 participants (“90%” stated in results table but the study authors reported “80%” in the article text) improved in self-rated assessments with 4/10 participants (40%) rating the improvement as major two weeks after BTX-A injections. With placebo, 4/10 participants (40%) still responded positively, with 1/10 participant (10%) noting major improvement. Generally, the authors of this study indicated that 5/10 participants (50%) performed better after BTX-A injections during functional performance testing. One participant was weaker, wrote faster and performed better with placebo injection. Overall 8/10 BTX-A participants (80%) reported improvement and 4/10 participants (40%) receiving placebo injections reported benefit.
Kruisdijk et al. [12] compared BTX-A treated WC participants to placebo. Some of the participants involved in this trial also had electrophysiological measurements that Contarino et al. [18] reported elsewhere. The primary outcome measure for this study was the participants’ answers to the following question: “Considering all advantages and disadvantages of this treatment, is the improvement such that you wish to continue this treatment or not?” The study authors found that 14/20 participants (70%) in the BTX-A group wished to continue treatment versus 6/19 participants (32%) in the placebo group (p=0.03). The study authors used an unpaired t-test to analyze the mean difference for functional performance measures which was calculated by subtracting the change in baseline to eight weeks with placebo from the change in baseline to eight weeks with BTX-A treatment. They reported a significant mean difference which was better with BTX-A regarding SSS, WCRS, writing speed (lines) and VAS for handwriting. The mean difference in the FSS was not significant. A subgroup analysis showed no evidence of treatment difference between the subgroups of participants with simple versus complex WC. The study authors reported that 20/39 participants (51%) received treatment for a year after completion of the study because of its benefit.
Writing speed was used in all three trials for assessing the participants. In Tsui et al. [10] the time is taken to complete copying a standard passage and the number of words per minute was apparently recorded but was not presented in that publication. They noted that writing speed improved in 7/20 participants (35%). The writing samples from the participants were randomized and given to a “blinded” physician for scoring on a scale of 0 to 3 (0 = totally illegible; 1 = some words illegible; 2 = all words legible, some with difficulty; and 3 = all words easily legible). In the study by Cole et al. [11] participants with WC wrote a standard paragraph, and the time required for its completion was recorded. The study authors reported that 4/6 participants (67%) with WC wrote faster after the BTX-A injections. Though individual writing speeds of the participants were provided, a summary statistical analysis was not presented in the paper. Kruisdijk et al. [12] conducted a test of writing speed by measuring the number of lines of a standard text written within two minutes. The mean difference in the writing speed between placebo and BTX-A from baseline to 8 weeks was 1.14 lines (95% CI, 0.07 to 2.20, p=0.04), better with BTX-A.
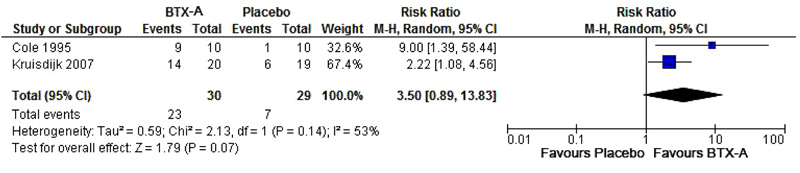
Two studies [11, 12] were included in our meta-analysis (Tsui et al. [10] did not report results in the placebo group and therefore could not be included in the meta-analysis). Combining the results of the self-rated improvements in these studies showed that the pooled effect estimate was not significantly different from placebo (p = 0.07) Fig. (2).
3.4. Safety
Muscle weakness after BTX-A injections was noted in participants in all three trials. In the trial by Tsui et al. [10], all 20 participants who had BTX-A experienced muscle weakness. Seven out of 10 participants (70%) in the trial by Cole et al. [11] had muscle weakness. Kruisdijk et al. [12] reported weakness in the hand in 18/20 participants (90%) in the BTX-A group and in 2/19 participants (11%) in the placebo group. In the study by Kruisdijk et al. [12] the authors reported that 1/20 participants (5%) in the BTX-A group and 3/19 participants (16%) in the placebo group reported pain at the injection site. Overall, 46/50 participants (92%) experienced weakness of the muscles injected, which appeared to be transient with spontaneous recovery. One participant, however, experienced weakness which lasted for eight days. Across the studies, 49 participants received placebo but results were only reported on 29 participants. Four of these 29 participants who received a placebo (14%) developed weakness. The study authors reported that the weakness and pain were temporary and participants recovered completely. Tsui et al. [10] noted that besides weakness in the participants who received BTX-A, no other adverse events or systemic adverse reactions were reported. Those participants who received placebo reported no adverse events. Cole et al. [11] also reported that BTX-A was generally “safe to use”. Kruisdijk et al. [12] reported that only 1/20 participants (5%) on BTX-A and 3/19 participants (16%) on placebo had pain at the injection site. No serious adverse events were reported in any of the studies. None of the participants in any of the three studies was reported to have withdrawn from the trials.

| First Author / Year | Participants with any Adverse Event | Participants with any Serious Adverse Event | Participants withdrawing due to Adverse Events |
|---|---|---|---|
| Tsui (1993) [10] | 20/20 participants had weakness after BTX-A injection. 12/20 had pain at site of injection. |
None reported | None reported |
| Cole (1995) [11] | 7/10 participants had muscle weakness with BTX-A. 2/10 had weakness with placebo. |
None reported | None reported |
| Kruisdijk (2007) [12] | 18/20 participants had weakness in the hand with BTX-A. 2/19 participants reported hand weakness with placebo. |
None reported | None reported |
4. DISCUSSION
This systematic review identified only three [10-12] published articles that evaluated BTX for FHD in randomized, double-blind, placebo-controlled trials.
4.1. Validated Measures of Improvement in Hand Function
Our primary outcome of interest in this systematic review was hand function, as measured by any validated hand function assessment at two weeks to two months post-treatment with BTX or placebo. In all three trials [10-12] the participants were evaluated within this primary time frame. Validated measures of hand function were used in two [10, 12] of the three trials. Tsui et al. [10] used Gibson’s maze as a validated measure of improvement in hand function. They noted that the errors made and the time to complete the maze improved from the baseline to two weeks and then at six weeks following BTX-A treatment. This improvement was found to be significant (p<0.01). No participant improved on placebo treatment with Gibson’s maze assessment. Kruisdijk et al. [12] used the WCRS, also a validated measure, scored on a scale from 0 to 16 points, to compare BTX-A treatment and placebo from baseline to eight weeks of treatment; they reported significant improvement in the WCRS, with a mean difference of -1.51 points (95% CI, -2.55 to -0.47, p < 0.01) in favor of BTX-A. The FSS and the SSS have been validated for use in carpal tunnel syndrome, but to our knowledge, neither these tools nor the VAS have been validated specifically for FHD. Cole et al. [11] used other dystonia inducing tasks and measured the improvement in hand function, but as far as we know, these measures have also not been validated. None of the publications reported on validated measures of hand function at two months to six months post-treatment.
4.2. Reduction in Symptoms
Another secondary outcome, reduction in symptoms associated with FHD, was investigated as part of this systematic review. Tremor and myoclonus were not described as characteristics of FHD in any participant pre-treatment. It should be noted that a reduction in pain may have been represented in the participant’s subjective assessments, but this was only explicitly described by Tsui et al. [10] and Kruisdijk et al. [12] Tsui et al. [10] found that 8/12 participants (67%) with pain improved with BTX-A and none with placebo on subjective assessment. Kruisdijk et al. [12] used the SSS to evaluate the direct and indirect symptoms of WC (pain, spasm, weakness, and numbness). Though they did not elaborate on each of these symptoms, the study authors demonstrated a statistically significant improvement in overall symptoms with a mean difference, -2.44 points, (95% CI, -4.52 to -0.37, p= 0.02) favoring BTX-A over placebo from baseline to eight weeks.
4.3. Quality of Life Measures
No specific quality of life measures were reported in any of the three trials.
4.4. Adverse Events
The intended therapeutic effect of BTX is to create weakness in the antagonist muscles but only to the extent that the dystonic symptoms are reduced. Weakness to the extent that it limits desired hand functions would be considered an adverse event. The three studies [10-12] had a total of 50 participants who received BTX-A injections. Overall, 46/50 participants (92%) experienced weakness of the muscles injected, which appeared to be transient with spontaneous recovery. Four out of 29 participants (14%) who received placebo developed weakness. Kruisdijk et al. [12] reported that only 1/20 participants (5%) on BTX-A and 3/19 participants (16%) who received placebo had pain at the injection site.
4.5. Limitations
The number of participants in all three studies was small, severely limiting any conclusions that can be drawn from our analysis. Only one study, Cole et al. [11] involved musicians and stenographers, a total of four such participants. This review is therefore essentially about WC. A major problem with research on BTX is that the nature of the treatment and the predictability of the weakness may make it impossible to adequately blind participants (and there are also challenges in blinding assessors).
4.6. Authors’ Conclusions
The number of participants included in this systematic review is too small to draw dependable conclusions for clinical practice about the efficacy and safety of BTX-A for FHD. There is currently not enough evidence to recommend the routine use of BTX-A for FHD, even though the treatment does show some promise. We would like to emphasize that this conclusion is reached because of a lack of dependable evidence for effectiveness derived from clinical trials, not because of evidence for lack of effectiveness of the intervention. As such, future studies are needed and our conclusions may need to be revisited when further quality evidence on sufficient numbers of patients is available.
4.7. Future Research Direction
There is a need for more and larger studies to evaluate BTX in FHD. In order to detect effect sizes ranging from 0.55 to 0.94 of having favorable outcomes between the intervention group receiving BTX and placebo or alternative treatment (as reported in Kruisdijk et al. [12]) a range of 19 to 53 participants per group needs to be recruited to attain a power of 80% with a type I error of 0.05.
The effectiveness of blinding participants and assessors could also be explored in future research on FHD. For example, future trials may report on the proportion of patients correctly guessing their treatment group allocation. Trials could be of either parallel group design or crossover design, and it would be informative to investigate if blinding is more effectively maintained in parallel group trials, where the study participants only encounter either the active intervention or placebo in the context of the study.
Also, adhering to a core set of outcomes as regards measures of both efficacy and safety is recommended, because it would aid the comparability of future studies and would also enable us to combine such studies in future meta-analyses. Specifically, we would recommend adhering to the efficacy outcomes detailed in this review, namely: hand function as measured by a validated tool at two weeks to two months post-treatment, a validated assessment of hand function at two to six months post-treatment, the secondary symptoms pain and tremor, and an assessment of quality of life using a validated tool. As regards safety outcomes, we recommend reporting on the numbers of participants experiencing any adverse event, any serious adverse event, or withdrawing due to an adverse event.
Future research should furthermore specifically address distinct occupational groups, such as musicians and stenographers, and stratify the results for the different task specific dystonias.
CONSENT FOR PUBLICATION
Not applicable.
CONFLICT OF INTEREST
The authors declares no conflict of interest, financial or otherwise.
ACKNOWLEDGEMENT
The authors are grateful to Dr. Tanya Jackson for editorial comments on the manuscripts.
DISCLOSURE STATEMENTS
This study was supported by a grant from the Workers’ Compensation Board of Alberta.
Dr. Nigel Ashworth reports no disclosures.
Dr. Henry Aidoo reports no disclosures.
Dr. David Antle reports no disclosures.
Dr. Alexander Doroshenko reports no disclosures.
Dr. Charl Els reports no disclosures.
Dr. David M. Flaschner reports no disclosures.
Dr. Douglas P. Gross reports no disclosures.
Dr. Christine Guptill reports no disclosures.
Dr. Patrick Potter reports no disclosures.
Ms. Maria C. Tan reports no disclosures.
Dr. Sebastian Straube has received funding for a program of research of which the present systematic review is part, from the Workers' Compensation Board of Alberta. Dr. Straube also declares honoraria from the Workers' Compensation Board of Alberta and from Oxford Medical Knowledge as well as advisory board fees from Daiichi Sankyo, Inc.
| # | Searches | Results |
|---|---|---|
| 1 | Dystonic disorders/ or (dystoni* or cramp*).mp. | 24899 |
| 2 | upper extremity/ or exp hand/ or (hand* or finger* or wrist* or phalanx or phalanges or digit or digits or digital or thumb* or palm* or digitorum or carpi or carpal* or metacarpal* or pollicis or interphalangeal or stenographer* or writer* or typist* or telegraphist* or musician* or clarinet* or flautist* or flutist* or flute or oboe or oboist* or saxophone or saxophonist* or bagpipe* or bassoon or bassoonist* or guitar or guitarist* or bass or bassist* or banjo* or harp or harpist* or zither* or mandolin* or koto* or string player* or violin or violinist* or viola or violist* or cello or cellist* or keyboard* or pianist* or piano* or organist* or harpsichord* or accordion* or percussion* or drummer* or tabla* or bata or brass or trumpet* or trombon* or tuba or hornplayer* or barber* or beautician* or golfer*).mp. | 1016570 |
| 3 | 1 and 2 | 2520 |
| 4 | ((focal and dystoni* and (limb* or extremit*)) or yips).ti,ab,kf. | 310 |
| 5 | 3 or 4 | 2707 |
| 6 | exp Botulinum toxins/ or exp Clostridium botulinum/ or (Botox or BTX or botulin* or Meditoxin or Neuronox or Oculinum or OnabotulinumtoxinA or Abobotulinumtoxina or Evabotulinumtoxina or Incobotulinumtoxina or Allergan or Azzalure or Bocouture or Botox or Dysport or Onaclostox or Toxine botulinique or Vistabel or Xeomin or rimabotulinum* or Myobloc or NeuroBloc or Bontoxilysin or Tentoxilysin or “Tetanus neurotoxin”).mp. | 23013 |
| 7 | 5 and 6 | 372 |
| 8 | randomized controlled trial.pt. | 482732 |
| 9 | clinical trial.pt. | 534532 |
| 10 | randomi?ed.ti,ab. | 541005 |
| 11 | placebo.ti,ab. | 202113 |
| 12 | dt.fs. | 2066830 |
| 13 | randomly.ti,ab. | 293378 |
| 14 | trial.ti,ab. | 520363 |
| 15 | groups.ti,ab. | 1829727 |
| 16 | or/8-15 | 4443267 |
| 17 | animals/ | 6372284 |
| 18 | humans/ | 17419632 |
| 19 | 17 not (17 and 18) | 4556224 |
| 20 | 16 not 19 | 3858878 |
| 21 | 7 and 20 | 244 |
| 22 | remove duplicates from 21 | 236 |
1974 to 2017 September 28
Interface - OVID
| # | Searches | Results |
|---|---|---|
| 1 | dystonia/ or dystonic disorder/ or (dystoni* or cramp*).ti,ab,kf. | 38576 |
| 2 | upper extremity/ or exp hand/ or (hand* or finger* or wrist* or phalanx or phalanges or digit or digits or digital or thumb* or palm* or digitorum or carpi or carpal* or metacarpal* or pollicis or interphalangeal or stenographer* or writer* or typist* or telegraphist* or musician* or clarinet* or flautist* or flutist* or flute or oboe or oboist* or saxophone or saxophonist* or bagpipe* or bassoon or bassoonist* or guitar or guitarist* or bass or bassist* or banjo* or harp or harpist* or zither* or mandolin* or koto* or string player* or violin or violinist* or viola or violist* or cello or cellist* or keyboard* or pianist* or piano* or organist* or harpsichord* or accordion* or percussion* or drummer* or tabla* or bata or brass or trumpet* or trombon* or tuba or hornplayer* or barber* or beautician* or golfer*).ti,ab,kf. | 1097218 |
| 3 | 1 and 2 | 4142 |
| 4 | exp focal hand dystonia/ or ((focal and dystoni* and (limb* or extremit*)) or yips).ti,ab,kf. | 1248 |
| 5 | 3 or 4 | 4584 |
| 6 | exp Botulinum toxins/ or exp Clostridium botulinum/ or (Botox or BTX or botulin* or Meditoxin or Neuronox or Oculinum or OnabotulinumtoxinA or Abobotulinumtoxina or Evabotulinumtoxina or Incobotulinumtoxina or Allergan or Azzalure or Bocouture or Botox or Dysport or Onaclostox or Toxine botulinique or Vistabel or Xeomin or rimabotulinum* or Myobloc or NeuroBloc or Bontoxilysin or Tentoxilysin or “Tetanus neurotoxin”).ti,ab,kf. | 33291 |
| 7 | 5 and 6 | 785 |
| 8 | exp clinical trial/ | 1262347 |
| 9 | randomi?ed.ti,ab. | 711891 |
| 10 | placebo.ti,ab. | 261316 |
| 11 | dt.fs. | 3484972 |
| 12 | randomly.ti,ab. | 365388 |
| 13 | trial.ti,ab. | 677385 |
| 14 | groups.ti,ab. | 2374964 |
| 15 | or/8-14 | 6529004 |
| 16 | animal/ | 1812089 |
| 17 | human/ | 18870014 |
| 18 | 16 not (16 and 17) | 1379198 |
| 19 | 15 not 18 | 6364607 |
| 20 | 7 and 19 | 505 |
| 21 | remove duplicates from 20 | 495 |
Interface - EBSCOhost Research Databases
Search Screen - Advanced Search
| # | Query | Results |
|---|---|---|
| S1 | (MH “Dystonic disorders“) OR (MH “Dystonia”) OR (dystoni* or cramp*) | 4,475 |
| S2 | (MH “Upper extremity”) OR (MH “Hand+”) OR (hand* or finger* or wrist* or phalanx or phalanges or digit or digits or digital or thumb* or palm* or digitorum or carpi or carpal* or metacarpal* or pollicis or interphalangeal or stenographer* or writer* or typist* or telegraphist* or musician* or clarinet* or flautist* or flutist* or flute or oboe or oboist* or saxophone or saxophonist* or bagpipe* or bassoon or bassoonist* or guitar or guitarist* or bass or bassist* or banjo* or harp or harpist* or zither* or mandolin* or koto* or string player* or violin or violinist* or viola or violist* or cello or cellist* or keyboard* or pianist* or piano* or organist* or harpsichord* or accordion* or percussion* or drummer* or tabla* or bata or brass or trumpet* or trombon* or tuba or hornplayer* or barber* or beautician* or golfer*) | 160,193 |
| S3 | S1 AND S2 | 496 |
| S4 | (MH “Focal hand dystonia”) OR ((focal and dystoni* and (limb* or extremit*)) or yips) | 168 |
| S5 | S3 OR S4 | 561 |
| S6 | (MH “Botulinum toxins”) OR (Botox or BTX or botulin* or Meditoxin or Neuronox or Oculinum or OnabotulinumtoxinA or Abobotulinumtoxina or Evabotulinumtoxina or Incobotulinumtoxina or Allergan or Azzalure or Bocouture or Botox or Dysport or Onaclostox or “Toxine botulinique” or Vistabel or Xeomin or rimabotulinum* or Myobloc or NeuroBloc or Bontoxilysin or Tentoxilysin or “Tetanus neurotoxin”) | 5,028 |
| S7 | S5 AND S6 | 82 |
| S8 | ((MH “Random Assignment”) or (MH “Random Sample+”) or (MH “Crossover Design”) or (MH “Clinical Trials+”) or (MH “Comparative Studies”) or (MH “Control (Research)+”) or (MH “Control Group”) or (MH “Factorial Design”) or (MH “Quasi-Experimental Studies+”) or (MH “Placebos”) or (MH “Meta-Analysis”) or (MH “Sample Size”) or (MH “Research, Nursing”) or (MH “Research Question”) or (MH “Research Methodology+”) or (MH “Evaluation Research+”) or (MH “Concurrent Prospective Studies”) or (MH “Prospective Studies”) or (MH “Nursing Practice, Research-Based”) or (MH “Solomon Four-Group Design”) or (MH “One-Shot Case Study”) or (MH “Pretest-Posttest Design+”) or (MH “Static Group Comparison”) or (MH “Study Design”) or (MH “Clinical Research+”)) or (clinical nursing research or random* or cross?over or placebo* or control* or factorial or sham* or meta?analy* or systematic review* or blind* or mask* or trial*) | 2,107,928 |
| S9 | S7 AND S8 | 46 |
Interface - EBSCOhost Research Databases
Search Screen - Advanced Search
| # | Query | Results |
|---|---|---|
| S1 | DE “DYSTONIA” OR DE “FOCAL dystonia” OR (dystoni* or cramp*) | 1,660 |
| S2 | DE “ARM” OR (DE “WRIST” OR DE “CARPAL bones” OR DE “WRIST extension” OR DE “WRIST flexion”) OR (DE “HAND” OR DE “FINGERS” OR DE “FLEXOR tendons” OR DE “METACARPUS”) OR (hand* or finger* or wrist* or phalanx or phalanges or digit or digits or digital or thumb* or palm* or digitorum or carpi or carpal* or metacarpal* or pollicis or interphalangeal or stenographer* or writer* or typist* or telegraphist* or musician* or clarinet* or flautist* or flutist* or flute or oboe or oboist* or saxophone or saxophonist* or bagpipe* or bassoon or bassoonist* or guitar or guitarist* or bass or bassist* or banjo* or harp or harpist* or zither* or mandolin* or koto* or string player* or violin or violinist* or viola or violist* or cello or cellist* or keyboard* or pianist* or piano* or organist* or harpsichord* or accordion* or percussion* or drummer* or tabla* or bata or brass or trumpet* or trombon* or tuba or hornplayer* or barber* or beautician* or golfer*) | 160,253 |
| S3 | S1 AND S2 | 160 |
| S4 | (focal and dystoni* and (limb* or extremit*)) or yips | 256 |
| S5 | S3 OR S4 | 388 |
| S6 | DE “BOTULINUM toxin” OR (Botox or BTX or botulin* or Meditoxin or Neuronox or Oculinum or OnabotulinumtoxinA or Abobotulinumtoxina or Evabotulinumtoxina or Incobotulinumtoxina or Allergan or Azzalure or Bocouture or Botox or Dysport or Onaclostox or “Toxine botulinique” or Vistabel or Xeomin or rimabotulinum* or Myobloc or NeuroBloc or Bontoxilysin or Tentoxilysin or “Tetanus neurotoxin”) | 930 |
| S7 | S5 AND S6 | 13 |
Interface - Wiley Interscience
Strategy applied to CDSR, DARE, CENTRAL
| ID | Search | Hits |
|---|---|---|
| #1 | [mh ^”Dystonic disorders”] or (dystoni* or cramp*):ti,ab,kw | 2607 |
| #2 | [mh ^”Upper extremity”] or [mh hand] or (hand* or finger* or wrist* or phalanx or phalanges or digit or digits or digital or thumb* or palm* or digitorum or carpi or carpal* or metacarpal* or pollicis or interphalangeal or stenographer* or writer* or typist* or telegraphist* or musician* or clarinet* or flautist* or flutist* or flute or oboe or oboist* or saxophone or saxophonist* or bagpipe* or bassoon or bassoonist* or guitar or guitarist* or bass or bassist* or banjo* or harp or harpist* or zither* or mandolin* or koto* or string player* or violin or violinist* or viola or violist* or cello or cellist* or keyboard* or pianist* or piano* or organist* or harpsichord* or accordion* or percussion* or drummer* or tabla* or bata or brass or trumpet* or trombon* or tuba or hornplayer* or barber* or beautician* or golfer*):ti,ab,kw | 45535 |
| #3 | #1 and #2 | 180 |
| #4 | ((focal and dystoni* and (limb* or extremit*)) or yips):ti,ab,kw | 11 |
| #5 | #3 or #4 | 190 |
| #6 | [mh “Botulinum toxins”] or [mh “Clostridium botulinum”] or (Botox or BTX or botulin* or Meditoxin or Neuronox or Oculinum or OnabotulinumtoxinA or Abobotulinumtoxina or Evabotulinumtoxina or Incobotulinumtoxina or Allergan or Azzalure or Bocouture or Botox or Dysport or Onaclostox or Toxine botulinique or Vistabel or Xeomin or rimabotulinum* or Myobloc or NeuroBloc or Bontoxilysin or Tentoxilysin or “Tetanus neurotoxin”):ti,ab,kw | 2800 |
| #7 | #5 and #6 | 34 |
Scopus
((TITLE-ABS-KEY (botox OR btx OR botulin* OR meditoxin OR neuronox OR oculinum OR
onabotulinumtoxina OR abobotulinumtoxina OR evabotulinumtoxina OR incobotulinumtoxina OR allergan OR
azzalure OR bocouture OR botox OR dysport OR onaclostox) OR TITLE-ABS-KEY (“Toxine botulinique” OR
vistabel OR xeomin OR rimabotulinum* OR myobloc OR neurobloc OR bontoxilysin OR tentoxilysin OR
“Tetanus neurotoxin”))) AND ((TITLE-ABS-KEY ((dystoni* OR cramp*) W/3 (hand* OR finger* OR wrist*
OR phalanx OR phalanges OR digit OR digits OR digital OR thumb* OR palm* OR digitorum OR carpi OR
carpal* OR metacarpal* OR pollicis OR interphalangeal))) OR (TITLE-ABS-KEY ((dystoni* OR cramp*) W/3
(stenographer* OR writer* OR typist* OR telegraphist*))) OR (TITLE-ABS-KEY ((dystoni* OR cramp*) W/3
(musician* OR clarinet* OR flautist* OR flutist* OR flute OR oboe OR oboist* OR saxophone OR
saxophonist* OR bagpipe* OR bassoon OR bassoonist* OR guitar OR guitarist* OR bass OR bassist* OR
banjo* OR harp OR harpist*))) OR (TITLE-ABS-KEY ((dystoni* OR cramp*) W/3 (zither* OR mandolin*
OR koto* OR “string player*” OR violin OR violinist* OR viola OR violist* OR cello OR cellist*))) OR (
TITLE-ABS-KEY ((dystoni* OR cramp*) W/3 (keyboard* OR pianist* OR piano* OR organist* OR
harpsichord* OR accordion* OR percussion* OR drummer* OR tabla* OR bata))) OR (TITLE-ABS-KEY ((
dystoni* OR cramp*) W/3 (brass OR trumpet* OR trombon* OR tuba OR hornplayer* OR barber* OR
beautician* OR golfer*))) OR (TITLE-ABS-KEY (“golf* Yips”))) AND ((TITLE-ABS-KEY (clinical W/2 research)
OR TITLE-ABS-KEY (random* OR cross?over OR placebo* OR control* OR factorial OR sham* OR
meta?analy* OR systematic AND review* OR blind* OR mask* OR trial*)))
PEDro
Setting: Basic Search
5 separate searches:
dystoni* AND hand*
dystoni* AND wrist*
dystoni* AND finger*
dystoni* AND musician*
cramp* AND musician*
Timespan = All years
| Set | Results | Search |
|---|---|---|
| #5 | 191 | #4 AND #3 DocType=All document types; Language=All languages; |
| #4 | 7,212,290 | TOPIC: (clinical NEAR/2 research) ORTOPIC: (random* OR cross?over OR placebo* OR control* OR factorial OR sham* OR meta?analy* OR systematic AND review* OR blind* OR mask* OR trial*) DocType=All document types; Language=All languages; |
| #3 | 379 | #2 AND #1 DocType=All document types; Language=All languages; |
| #2 | 26,825 | TOPIC: (Botox or BTX or botulin* or Meditoxin or Neuronox or Oculinum or OnabotulinumtoxinA or Abobotulinumtoxina or Evabotulinumtoxina or Incobotulinumtoxina or Allergan or Azzalure or Bocouture or Botox or Dysport or Onaclostox Or “Toxine botulinique” or Vistabel or Xeomin or rimabotulinum* or Myobloc or NeuroBloc or Bontoxilysin or Tentoxilysin or “Tetanus neurotoxin”) DocType=All document types; Language=All languages; |
| #1 | 1,932 | TOPIC: ((dystoni* or cramp*) NEAR/3 (hand* or finger* or wrist* or phalanx or phalanges or digit or digits or digital or thumb* or palm* or digitorum or carpi or carpal* or metacarpal* or pollicis or interphalangeal)) OR TOPIC: ((dystoni* or cramp*) NEAR/3 (stenographer* or writer* or typist* or telegraphist* or musician* or clarinet* or flautist* or flutist* or flute or oboe or oboist* or saxophone or saxophonist* or bagpipe* or bassoon or bassoonist* or guitar or guitarist* or bass or bassist* or banjo* or harp or harpist* or zither* or mandolin* or koto* or “string player*” or violin or violinist* or viola or violist* or cello or cellist* or keyboard* or pianist* or piano* or organist* or harpsichord* or accordion* or percussion* or drummer* or tabla* or bata or brass or trumpet* or trombon* or tuba or hornplayer* or barber* or beautician* or golfer*)) OR TOPIC: (“golf* Yips”) DocType=All document types; Language=All languages; |
Clinicaltrials.gov
Setting - Advanced Search
Condition/Disease:
“Focal hand dystonia” OR (focal AND dystonia AND (wrist OR hand OR finger OR thumb OR limb OR limbs OR extremity OR extremities)) OR ((writer OR stenographer OR typist OR keyboard OR piano OR musician OR barber OR golfer) AND (dystonia OR cramp))
AND
Intervention/Treatment:
Botox OR botulinum OR Meditoxin OR Neuronox OR Oculinum OR OnabotulinumtoxinA OR Abobotulinumtoxina OR Evabotulinumtoxina OR Incobotulinumtoxina OR Allergan OR Azzalure OR Bocouture OR Dysport OR Vistabel OR Xeomin OR Myobloc OR NeuroBloc OR Bontoxilysin
International Clinical Trials Registry Portal (ICTRP)
Setting - Advanced Search
28 Sep 2017
Botox OR botulinum OR Meditoxin OR Neuronox OR Oculinum OR OnabotulinumtoxinA OR Abobotulinumtoxina OR Evabotulinumtoxina OR Incobotulinumtoxina OR Allergan OR Azzalure OR Bocouture OR Dysport OR Vistabel OR Xeomin OR Myobloc OR NeuroBloc OR Bontoxilysin AND dystoni* AND focal OR wrist OR hand OR finger OR thumb OR limb OR limbs OR extremity OR extremities OR writer OR stenographer OR typist OR keyboard OR piano OR musician OR barber OR golfer OR cramp* AND focal OR wrist OR hand OR finger OR thumb OR limb OR limbs OR extremity OR extremities OR writer OR stenographer OR typist OR keyboard OR piano OR musician OR barber OR golfer
| First Author/Year | Reasons for
Exclusion |
|---|---|
| Contarino (2007) [18] | Reported on a subset of participants that were also included in Kruisdijk (2007) [12] |
| Wissel (1996) [19] | Not a randomized controlled trial |
| Jabusch (2005) [20] | Not a randomized controlled trial |
| Lungu (2011) [21] | Not a randomized controlled trial |
| Bares (2002) [22] | Not a randomized controlled trial |
| Behari (1998) [23] | Case series |
| Berg (1999) [24] | Not a randomized controlled trial |
| Dashtipour (2015) [25] | Systematic review |
| Grigoriu (2015) [26] | Systematic review |
| Dresel (2014) [27] | Did not include botulinum toxin |
| Ross (1997) [28] | Not a randomized controlled trial |
| Childers (1998) [29] | Systematic review |
| Cohen (1989) [30] | Not a randomized controlled trial |
| Dashtipour (2008) [31] | Systematic review |
| Zeuner (2013) [32] | Not a randomized controlled trial |
| Djebbari (2004) [33] | Not a randomized controlled trial |
| Jankovic (1993) [34] | Not a randomized controlled trial |
| Schuele (2003) [35] | Not a randomized controlled trial |
| Rivest (1991) [36] | Not a randomized controlled trial |
| Yoshimura (1992) [37] | Trial included participants who did not have focal hand dystonia |
| Chen (1999) [38] | Participants had concurrent intervention of either resting or exercising the injected muscles |


