All published articles of this journal are available on ScienceDirect.
Neuropsychological and Syntactic Deficits in HIV Seropositive Males
Abstract
Objective:
Given the underlying frontal-basal ganglia circuit neuropathogenesis of HIV-infected individuals, it is surprising that little is reported about potential language deficits as part of their higher cognitive dysfunctional profile. This study aims to elucidate whether HIV-positive individuals have linguistic impairments that may originate from or be intensified by deficits in cognitive functions. The research questions address (i) quantitative differences in sentence repetition abilities involving complex syntactic phenomena between adults with HIV and non-HIV healthy controls (ii) correlations of sentence repetition scores with neurocognitive measures and (iii) correlation of sentence repetition performance with duration and severity of HIV.
Methods:
A battery of neuropsychological tests were administered to 40 HIV - seropositive males and 40 demographically matched healthy controls to assess verbal learning/episodic memory, psychomotor speed, executive functions and visuospatial abilities. Language abilities were evaluated using a repetition task that screened specific complex syntactic operations at the sentence-level.
Results:
A significant difference was noted between the two groups regarding correct repetition of the sentence repetition task with the control group outperforming the HIV-seropositive group. For the HIV group, significant correlations were found for correct sentence repetition with years of education, duration of illness, Mini-Mental State Examination, semantic and phonemic fluency, symbol digit modality test scores, and the Trail Making Test (parts A and B).
Conclusion:
Speech-language pathologists and neuropsychologists should screen for language deficits associated with the different clinical syndromes in HIV patients as part of their routine clinical care.
1. INTRODUCTION
Globally around 40 million people are infected with the human immunodeficiency virus (HIV) [1]. Furthermore, the prevalence and implications of HIV-related neurocognitive impairment (NCI) remain poorly understood in clinical and research practice even across European Union member states [2, 3]. The aim of the current paper is to shed light on neurocognitive and linguistic deficits in HIV-positive men who have sex with men (MSM), the largest demographic group affected by HIV in Europe [4], using a multidisciplinary approach.
Given the underlying frontal-basal ganglia circuit neuropathogenesis of HIV, this study aimed to elucidate whether HIV-positive individuals have syntactic language impairments that may originate from or be intensified by deficits in neurocognitive functions as related to the severity of the disease [5]. One way to describe the link between cognitive domains and language abilities after HIV onset is to examine the statistical associations between individuals’ performance on neurocognitive and language assessment measures and compare this to non-infected healthy age and education-matched controls. Cognitive abilities were assessed on a wide range of tasks in specific domains such as verbal episodic memory, psychomotor speed, verbal fluency, visuospatial abilities and executive functions. Syntactic language abilities were screened using an offline repetition task involving complex structural operations at the sentence-level.
The research was carried out in Greece and is the first study to report on associated linguistic deficits within the neurocognitive profile of individuals with confirmed HIV seropositivity. The rationale spurring the research is to extend the information in this field by adding data on NCI for Greek-speaking HIV-infected groups and to advocate routine screening of high-level language as an additional biomarker of NCI [6-8]. Given that the HIV infection targets the central nervous system in frontal and subcortical areas [9-13], the present study used a quick measure of syntactic ability [14] to analyze complex language structures for Greek.
Sentence repetition is gaining increasing attention as a source of information about sentence-level language abilities for research and for clinical assessment purposes in developmental and acquired language deficits [14-16]. The general purpose of a sentence repetition test (SRT) is to evaluate the effects of different types of long-term linguistic knowledge on immediate recall. The ability to repeat auditory-verbal information using elicited imitation involves several cognitive processes, from auditory processing and phonological analysis to output mapping and speech production [17, 18].
The key research questions are as follows:
1. Is the performance of the HIV group different from the healthy control group on a battery of neurocognitive tasks?
2. Are there differences between the HIV-positive and control groups on a sentence repetition task (SRT) measuring higher-level language abilities (e.g., syntactic complexity)?
3. Can potential linguistic deficits in the HIV group be associated with neurocognitive abilities and/or stage of the disease (e.g., chronicity and/or severity) and/or level of education or age?
4. Which measures best predict group membership (HIV vs. control)?
2. METHODS
2.1. Participants
Seventy-three seropositive males attending the outpatient infectious disease units of two University hospitals in Greece; the Laiko Hospital in Athens and the University of Patras, Medical Hospital in Patras, were recruited to participate in the research. However, during the preliminary stage, thirty- three individuals were excluded because they were found to have either condition that could confound neurocognitive outcomes, alcohol or drug abuse or missing medical records. The remaining forty (40) served as the clinical research group for this study. Each participant provided informed consent, had completed at least junior high school, and was able to speak, read and understand Greek. In all cases, the route of transmission was MSM (men who have sex with men) and HIV infection was confirmed by standard clinical pathology laboratory testing (e.g., positive HIV antibodies detected by Elisa and confirmed with Western blot). The severity of HIV disease was assessed for each individual using the CDC (Center for Disease Control and Prevention) classification stages. Furthermore, no participant with HIV was positive either for the hepatitis C virus or for the hepatitis B virus. All the participants were on antiretroviral medication (i.e., HAART: Highly Active Antiretroviral Therapy), and were 3having successful treatment. Since all the participants were male homosexuals, the group is considered a homogeneous single-cohort group. Finally, no individual reported significant limitations with activities of daily living. HIV descriptors of the research group are reported in Table 1.
| – | HIV – positive males (n = 40) | |
|---|---|---|
| Diagnosis | Year Range | 1 - 16 |
| Mean (SD) | 5.78 (4.34) | |
| CDCa Stage: A; B; C | A (n=25), B (n=7), C (n=8) | |
| %HIV RNAb (plasma) | <50 UI/ml | |
| Current CD4c | 748.92 (295.31) | |
| CD4CD8d | 21.72 (41.7) | |
| Nadir CD4 | 356.05 (155.05) | |
| HCVe positive | No (n =40) | |
| HBVf positive | No (n =40) | |
Forty (20 males, 20 females) demographically matched healthy controls were also recruited from two large urban centers e.g., Southwestern Greece and Epirus (sample of convenience) and requested to take part in the study by the participating clinicians. They were native Greek speakers who provided written consent to participate. Males and females did not significantly differ with respect to age and education. There were no differences between the control group and the HIV-positive group on age and educationi. It is acknowledged that the sex differences within the control group as compared to the experimental group do not meet expectations for an experimentally equivalent control group for gender, but it was the best available comparison group for the battery of assessments of interest. Prior to the study, both the groups of participants completed the Greek version of the Hospital Anxiety and Depression Scale (HADS) to determine the levels of anxiety and depression that an individual may be experiencing. The HADS comprises fourteen items: seven items measure symptoms of anxiety (HADS-A) and seven items measure symptoms of depression (HADS-D). There was no significant difference between the overall score on the HAD between the HIV-positive and the control group. Nevertheless, it is probable that some individuals in the HIV group showed signs of mild depression but HIV-positive individuals with moderate-severe depression were excluded. Participants’ demographic characteristics are presented in Table 2.
| – | HIV-Positive | Controls | Significant (2-tailed) |
|---|---|---|---|
| Gender Male Female |
40 - |
20 20 |
|
| Age (years) Age Range Mean Age |
20 – 55 36.33 (8.48)a |
19 – 59 34.85 (9.96)a |
a p = 0.478 |
| Education (years) Range Mean |
9 – 23 14.58 (3)a |
11 – 20 14.98 (2.13)a |
a p = 0.494 |
| Employment (number) Employed Unemployed HADSb (total) |
35 5 11.88 (6.89)a |
36 4 9.45 (4.77)a |
a p = 0.07c |
2.2. Materials
A battery of neuropsychological tests were administered to assess cognitive status including global cognitive deficits, verbal learning/memory, visual scanning/psychomotor speed, executive functions and visuospatial abilities. The neuro- psychological tests were chosen with the intention of assessing cognitive domains found in recent studies [19-24] to be affected in HIV seropositive individuals. Moreover, these tests were adapted and standardized for the Greek population in an earlier work [25-29]. Each participant was tested individually on all measures in 2-3 sessions of 45 minutes each time. For the HIV-positive individuals, this was done in a quiet room in the hospital clinics. For the control group, testing was carried out in a quiet area of the participant’s home. The neurocognitive tests were administered by trained certified clinicians (psychologists and speech-language therapists) and scored by doctoral-level clinical neuropsychologists according to published administration instructions and norms provided in the test manuals. The Greek version of the MMSE [30], a 30-item brief screening measure for global cognitive deficits was administered to rule out dementia. The test examines eight different cognitive areas including orientation to time, orientation to space, attention, calculation, recall, language, repetition and complex commands. The Greek adaptation of the Rey Auditory Verbal Learning Test (RAVLT) [24] was administered to assess verbal learning and memory. The administration procedure was as follows: first Learning Trials 1-V, Trial B, and then Trial VI, were administered using a presentation rate of one word per second. A delayed recall trial was administered 25 minutes after completing Trial VI. A 50-word list recognition trial which includes the words of learning trials A and B and 20 distractor words was then given asking the participants to identify both List A and List B words and to indicate to which list they belong . The recognition score was the number of List A words correctly identified. The dependent variables included the mean number of words recalled on each trial I-V across trials for I-V (RAVLT total), and delayed recall. The Greek Trail Making Test Part A (Trails A) [28] was administered as an index of visual scanning as well as learning and psychomotor speed. In this task, the participants had to connect encircled numbers in ascending order as quickly as possible. The dependent variable included the time (in seconds) to complete Trails A. The Greek Trail Making Test Part B (Trails -B) [28] was administered to assess executive functioning. In this task, the participants were asked to connect encircled numbers, alternating between numbers and letters, in ascending order as quickly as possible. The dependent variable included the time (in seconds) to complete Trails B.
The Symbol Digit Modalities Test (SDMT) was used to measure mental processing speed and working memory. The SDMT is a substitution task in which participants, by using a reference key, have 90 seconds to pair specific numbers with given geometric figures. The detailed description of the original SDMT is available in Smith’s Clinical Manual [31], whereas the corresponding Greek norms can be found in the study by Argirokastritou et al. [32]. The Greek version of the SNST was used to measure executive functions [26]. The SNST measures response inhibition and attention. It consists of two tasks (colour task and colour-word task). Both the tasks include 112 colour names (e.g., red, green, blue, and brown, arranged in 4 columns of 28 names. In the colour task, participants were asked to read all the words aloud as quickly as possible, starting from the top of the first column. In the colour-word task, participants were asked to read aloud the colour in which the word was printed. The score was calculated by the number of correct responses. Norms were based on the results presented by Zalonis et al. [29]. The Line Orientation test was used to measure visuospatial ability. Patients were presented with a page containing numbered lines oriented in various directions (top of the page) and two lines oriented in various directions (bottom of page). They were then asked to correctly match (indicate by pointing or saying the respective number) the two bottom lines with two of the top lines in terms of orientation. The task has a series of 10 items, and the individual is given 20 seconds to complete each item. It is one of the tests included in the Repeatable Battery for the Assessment of Neuropsychological Status (RBANS) which assesses Immediate Memory, Visuospatial/Constructional abilities, Language, Attention, and Delayed Memory. The RBANS consists of 12 subtests, which yield 5 Index scores and a Total Scale score. Each index is composed of at least two tasks that are similar in content to popular neuropsychological measures. The Visuospatial/Constructional Index includes tasks similar to the Judgment of Line Orientation and Rey Complex Figure Copy tests [25]. To measure verbal fluency and lexical retrieval, the verbal fluency task was used. This task has two components, a semantic fluency test, and a phonemic fluency test. In the semantic fluency task, each participant was asked to generate in spoken form as many words possible from three semantic categories (objects, animals and fruits). In the phonological fluency task, participants were asked to orally produce as many words as possible that begin with the three distinct phonemes (Χ, Α, Σ). All the participants were allowed sixty seconds for each category. Repetitions of the same word as well as the production of proper names, people, and places were excluded from the data analysis. For every semantic category, and separately for every phoneme, the total number of the words produced was calculated to extract the average semantic fluency level of each participant. To screen syntactic abilities or morphosyntactic knowledge, a sentence repetition test (SRT) was used to analyse complex language structures for Greek using sentence-level probes [16]. The SRT consists of long sentences that disallow the passive echoing of the stimulus structure, and the participant can correctly repeat only if he/she has fully acquired the specific elicited structure. The task has 32 sentences targeting 8 different structures (four sentences per structure) including subject-verb-object (SVO), negation, wh-questions, coordination, relative and complement clauses. In terms of Greek language-specific structures, the SMG-SRT also includes adverbial clauses, and clitic left dislocation or clitic doubling (CLLD / CLD). Two of the negation structures include the negative particle min ‘not’ introduced by the particle na ‘to’, while the other two include the den ‘not’ particle in main clauses. Three of the indirect wh-questions include the question word ti ‘what’ and the question word pio ‘which’. All coordination structures include the coordinating conjunction ke ‘and’. The relative clauses start with the relative pronoun pu ‘who’ referring to the object of the main clause. Of the complement clauses, two are introduced by the pu element, one attached to a preceding verb and one to a noun, while the other two by the oti element, both attached to a preceding verb. All adverbial clauses report time including the conjunctions afu ‘since’, eno ‘while’, prin ‘before’ and otan ‘when’. Finally, two structures include clitic left dislocation and clitic doubling each. The SRT was administered by a certified speech and language therapist trained on the tool by the first author. The sentences varied in length from 9 to 12 words, and from 17 to 23 syllables and were presented in a fixed order across both groups of participants. Clarifications were provided by the experimenter regarding the procedure of the task, so that each participant needed to repeat the sentence heard, as loudly, and accurately as possible. All responses from each participant were recorded allowing detailed scoring after the completion of each testing session. The SRT was scored and analysed based on the audio-taped samples by two qualified psycholinguists from different universities who were blind to group allocation. Inter-rater reliability was calculated in 30% of the responses for sentence repetition and syntactic structure repetition respectively and it was high at 99.6%. First, every accurately repeated sentence scored 1 point and every inaccurate, incomplete, or incorrectly repeated sentence received no points. This allowed the calculation of the overall correct sentence repetition performance of each participant. Second, every accurately generated targeted-structure scored 1 point and every inaccurate repetition of the target structure scored no points. Third, all the types of syntactic errors (e.g., omissions, substitutions, additions, word order errors), for both content and function words were calculated.
2.3. Statistical Analysis
All data points that were considered extreme outliers were excluded from the analysis. The normality assumption for each variable was tested using the Shapiro-Wilk test. Even though some variables violated the normality assumption, we proceeded with the parametric independent samples t-test to examine the differences between the two groups (HIV seropositive and control group). The assumption of homogeneity of variances was tested via Levene’s F test for equality of variances. In case where the homogeneity assumption was violated, the Welch t-test was used instead. The effect size was estimated via Cohen’s d based on Cohen’s guidelines. Also, since the control group was mixed-sex and the HIV group had only male participants, a sensitivity analysis was performed using only the male participants of the control group. The analysis revealed that the differences in the whole control group and in only the male participants were insignificant. Pearson’s r-correlation coefficient was used to test the relationship between neuropsychological and linguistic assessments with chronological age, years of education, years post-diagnosis and syntactic performance between the variables. The level of statistical significance was set at α = .05 and all the analyses were conducted using SPSS 22.0 software.
3. RESULTS
Participants’ compliance rate was high, with no patient refusing to participate in any of the tasks. To address the research questions, the performance of the HIV-positive and healthy adult control groups was analysed for neurocognitive performance based on neuropsychological testing and for complex (syntactic) language abilities using a sentence repetition measure. There were no differences between male and female controls performance on the neuropsychological battery described in the Methods section (MMSE: p = 0.938; Semantic Fluency: p = 0.534; Phonemic Fluency: p = 0.665; SDMT: p = 0.924; TMTA: p = 0.184; TMTB: p = 0.159; LOT: p = 0.184; Stroop: p = 0.656; HADS Total: p = 0.439), hence we consider them one control group. Table 3 reports the performance for each group on the neuropsychological measures and the sentence repetition test.
| – | HIV-positive (n=40) | Controls (n=40) | – | – | – | – | ||
|---|---|---|---|---|---|---|---|---|
| – | M | SD | M | SD | t | df | p | d |
| MMSEa | 28.43 | 1.80 | 29.45 | 0.78 | -3.30 | 53.31 | .002k | 0.51 |
| RAVLTb Total | 49.58 | 9.34 | 55.53 | 7.54 | -3.13 | 78 | .002k | 0.49 |
| RAVLT1c | 6.40 | 1.95 | 7.53 | 1.60 | -2.82 | 78 | .006k | 0.44 |
| RAVLT 2c | 8.98 | 2.29 | 10.33 | 1.65 | -3.02 | 78 | .003k | 0.47 |
| RAVLT 3c | 10.35 | 2.48 | 11.65 | 2.14 | -2.51 | 78 | .014k | 0.39 |
| RAVLT 4c | 11.48 | 2.17 | 12.73 | 1.81 | -2.79 | 78 | .007k | 0.44 |
| RAVLT 5c | 12.40 | 2.05 | 13.30 | 1.70 | -2.14 | 78 | .036k | 0.33 |
| RAVLT Delayd | 10.68 | 2.57 | 12.13 | 2.34 | -2.63 | 78 | .010k | 0.41 |
| SDMTe | 44.35 | 11.70 | 53.83 | 10.05 | -3.88 | 78 | .000k | 0.61 |
| LOTf | 7.60 | 2.07 | 7.83 | 1.60 | -0.54 | 78 | .588 | 0.08 |
| SNSTg | 110.70 | 2.72 | 111.45 | 1.65 | -1.49 | 78 | .140 | 0.23 |
| TMTAh | 39.63 | 12.14 | 39.75 | 17.26 | -0.04 | 78 | .970 | 0.01 |
| TMTBi | 80.15 | 38.05 | 70.35 | 54.98 | 0.93 | 78 | .357 | 0.14 |
| Semantic fluency | 57.25 | 12.16 | 69.53 | 13.62 | -4.25 | 78 | .000k | 0.67 |
| Phonemic fluency | 36.25 | 15.47 | 46.28 | 11.97 | -3.24 | 78 | .002k | 0.51 |
| Sentence repetitionj | 27.20 | 3.50 | 30.00 | 1.59 | -4.60 | 54.33 | .000k | 0.72 |
| Syntactic structurej repetition | 30.73 | 2.14 | 31.63 | 0.62 | -2.55 | 45.68 | .014k | 0.41 |
| – | HIV-positive (n=40) | Controls (n=40) | – | – | – | – | ||
|---|---|---|---|---|---|---|---|---|
| – | M | SD | M | SD | t | df | p | d |
| SVOa | 3.98 | 0.16 | 4.00 | 0 | -1 | 39 | .323 | 0.13 |
| Negation | 3.7 | 0.6 | 3.98 | 0.16 | -2.77 | 44.25 | .008c | 0.45 |
| CLD-CD | 3.55 | 0.89 | 3.88 | 0.4 | -2.07 | 53.98 | .043c | 0.33 |
| Coordination | 4.00 | 0 | 3.95 | 0.22 | 1.43 | 39.00 | .160 | 0.22 |
| Complement Clauses | 3.7 | 0.69 | 3.95 | 0.22 | -2.19 | 46.96 | .033c | 0.34 |
| Adverbials | 3.9 | 0.3 | 3.95 | 0.22 | -.84 | 78 | .402 | 0.13 |
| Relative Clauses | 3.95 | 0.22 | 3.95 | 0.22 | 0 | 78 | 1.000 | 0 |
| Wh-questions | 3.95 | 0.22 | 3.98 | 0.16 | -.58 | 78 | .562 | 0.11 |
3.1. Cognitive Functions
Considering Table 3, the control group performed significantly better than the HIV-positive individuals in the MMSE (t(53.31) = -3.30, p = .002, d = 0.51), RAVLT total recall trial (total verbal learning) (t(78) = -3.30, p = .002, d = 0.49), Learning trials 1 (t(78) = -2.82, p = .006, d = 0.44), 2 (t(78) = -3.02, p = .003, d=0.47), 3 (t(78) = -2.51, p = .014, d = 0.39), 4 (t(78) = -2.79, p = .007, d = 0.44), 5 (t(78) = -2.14, p = .010, d = 0.33), delay recall (t(78) = -3.88, p < .001, d = 0.41) and in the SDMT (t(78) = -3.88, p = < .001, d = 0.61). Furthermore, in the verbal fluency measures, the HIV-positive group achieved a significantly lower verbal fluency score on both the semantic (t(78) = -4.25, p = < .001, d = 0.67) and the phonetic (t(78) = -3.24, p = .002, d = 0.51) subtests compared to the control group.
3.2. Sentence Repetition Performance
A significant difference (t(54.33) = -4.60, p < .001, d = 0.72) was observed between the HIV and control groups with respect to the correct (accurate) repetition of the stimuli-sentences; with the control group performing significantly higher than the HIV-positive group. Moreover, controls performed significantly better than the HIV-positive individuals (t(45.68) = -2.55, p = .014, d = 0.41) in correct repetition of the targeted structure embedded in each stimuli-sentence. Overall, the performance of the groups on the SRT for each targeted structure per sentence, reported in Table 4, revealed a significant difference for structures involving negation (t(44.25) = -2.77, p = .008, d = 0.45), the clitic left dislocation/clitic doubling (CLD-CD) structures (t(53.98) = -2.07, p = .043, d = 0.33) and the complement clauses (t(46.46) = -2.19, p = .033, d = 0.34) with the control group scoring significantly higher than the HIV group.
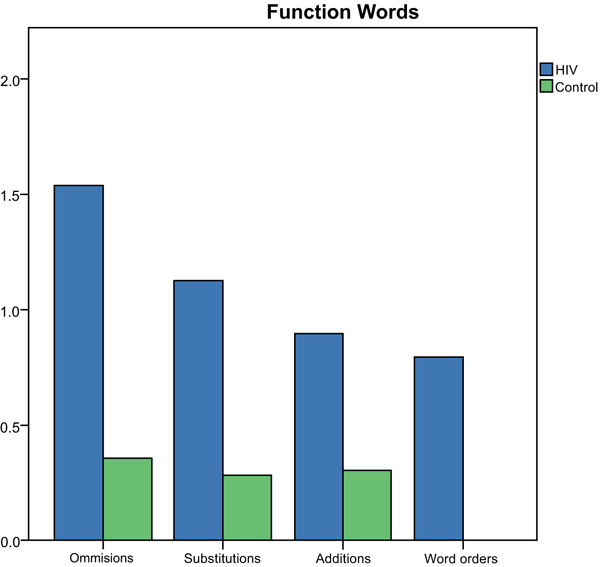
All word errors committed on the SRT by both the groups were divided into omissions, substitutions, additions, word order errors, for both the functions (e.g., prepositions, auxiliaries, quantifiers, pronouns) and content words (e.g., nouns, verbs, adverbs, adjectives). The total number of different error types for function words produced by each group is depicted in Fig. (1).
Number (y-axis) and type of errors (x-axis) produced for function words in the SRT by the group.
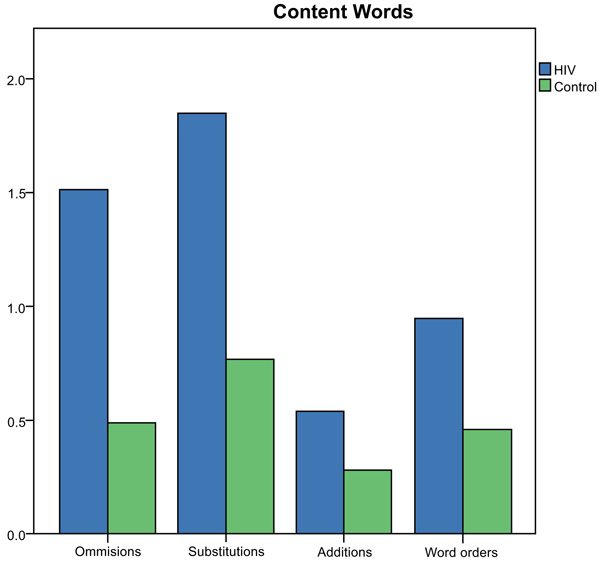
Results of the Mann-Whitney U Test indicated significantly more omission (z=-2.28, p=0.02), substitution (z=-3.93, p=0.00), addition (z=2.68, p=0.01) and word order errors (z=3.73, p=0.00) were produced by the HIV-group compared to controls for function words on the SRT. The total number of different error types for content words produced by each group is depicted in Fig. (2).
| – | Age | Education | Diagnosis | Sentence repetitioni | Syntactic repetitioni |
|---|---|---|---|---|---|
| Age | – | – | – | -0.24 (p=.136) | -0.049 (p=.765) |
| Education | – | – | – | 0.523j (p=.001) | 0.453j(p=.003) |
| Diagnosis | – | – | – | -0.378 (p=.016) | -0.455j(p=.003) |
| MMSEa | -.033 (p=.840) | 0.572j (p<0.001) | -0.303 (p=.057) | 0.386j (p=.014) | 0.512j(p=.001) |
| RAVLT Totalb | -.257 (p=.110) | 0.151 (p=.354) | -0.469j (p=.002) | 0.186 (p=.250) | 0.184 (p=.255) |
| RAVLT Delayc | -.344j(p=0.30) | 0.314j (p=.048) | -0.541j(p<.001) | 0.207 (p=.200) | 0.175 (p=.280) |
| SDMTd | -.204 (p=.207) | 0.605j (p<.001) | -0.336j (p=.034) | 0.426j(p=.006) | 0.451j (p=.003) |
| LOTe | -.100 (p=.538) | 0.351j (p=.026) | -0.432j(p=.005) | 0.071 (p=.662) | 0.154 (p=.343) |
| SNSTf | .311 (p=.051) | -0.148 (p=.363) | 0.016 (p=.923) | -0.19 (p=.240) | -0.059 (p=.719) |
| TMTAg | .309 (p=.052) | -0.398 (p=.011) | 0.478j(p=.002) | -0.328j(p=.039) | -0.364j (p=.021) |
| TMTBh | .298 (p=.062) | -0.484j (p=.002) | 0.426j(p=.006) | -0.384j(p=.014) | -0.421j (p=.007) |
| Semantic fluency | -0.171 (p=.291) | 0.326j(p=.040) | -0.039 (p=.810) | 0.352j(p=.026) | 0.231 (p=.152) |
| Phonemic fluency | -0.004 (p=.980) | 0.545j (p<.001) | -0.442j(p=.004) | 0.351j(p=.027) | 0.434j(p=.005) |
| CD4 | -0.053 (p=.747) | 0.328j(p=.042) | -0.045 (p=.784) | 0.164 (p=.320) | 0.147 (p=.373) |
| CD4 nadir | -0.324j (p=.047) | 0.122 (p=.465) | -0.172 (p=.303) | -0.113 (p=.501) | -0.137 (p=.412) |
Number (y-axis) and type of errors (x-axis) produced for content words on the SRT by the group.
Results of the Mann-Whitney U Test indicated significantly more word substitution (z=-3.29, p=0.00) and addition (z=2.15, p=0.02) errors were produced by the HIV-group compared to controls on the SRT for content words. There were non-significant differences between the groups for omission (z=-1.77, p=0.08) and word order (z=-.497, p=0.62) errors on the SRT.
3.3. Correlations between Measures
Performance measures for the HIV-positive cohort were carried out to investigate the relationship (or the contribution of) between neuropsychological and linguistic assessments with chronological age, years of education, years post-diagnosis and syntactic performance. The analysis was carried out using Pearson’s r-correlation coefficient and the results are presented in Table 5.
Regarding chronological age, we observed a significantly negative correlation with RAVLT delay scores (r(40) = -0.344, p = .030) and CD4 nadir scores (r(39) = -0.324, p = .047). In contrast, years of education revealed a significantly positive correlation with respect to MMSE (r(40) = 0.572, p < .001), RAVLT delay (r(40) = 0.314, p = .048), SDMT (r(40) = 0.605, p <0.001), LOT (r(40) = 0.351, p = .026), semantic fluency (r(40) = 0.326, p =.040), phonemic fluency (r(40) = 0.545, p <0.001) and CD4 (r(40) = 0.328, p =0.042). However, a negative correlation was observed with respect to the TMTA (r(40) = -0.398, p = .011) and the TMTB (r(40) = -0.484, p = 0.002). Allowing for the years post HIV diagnosis, we noticed a significantly negative correlation with RAVLT total scores (r(40) = -0.469, p = .002), RAVLT delay scores (r(40)= -0.541, p < .001), SDMT (r(40) = -0.336, p = 0.034), LOT (r(40) = -0.432, p = 0.005) and phonemic fluency (r(40) = -0.442, p = 0.004). Yet, significant positive correlations were observed with TMTA (r(40) = 0.478, p = 0.002) and TMTB (r(40) = 0.426, p = 0.006). Taking into account correct sentence repetition, we observed a significantly positive correlation with years of education (r(40) = 0.523, p = 0.001), MMSE scores (r(40) = 0.386, p = 0.014), SDMT (r(40) = 0.426, p = 0.006), semantic fluency (r(40) = 0.352, p = 0.026) and phonemic fluency scores (r(40) = 0.351, p = 0.027). Significant negative correlations were observed with duration of illness (r(40) = -0.378, p = 0.016), TMTA (r(40) = -0.328, p = 0.039) and (r(40) = 0.351, p = 0.027). Finally, for correct repetition of target syntactic structures, we report significantly positive correlations with years of education (r(40) = 0.453, p = 0.003), MMSE scores (r(40) = 0.512, p = 0.001), SDMT (r(40) = 0.451, p = 0.003) and phonemic fluency scores (r(40) = 0.434, p = 0.005). Significantly negative correlations were observed with duration of illness (r(40) = -0.455, p = 0.003), TMTA (r(40) = -0.364, p = 0.021) and TMTB (r(40) = -0.421, p = 0.007).
A binomial logistic regression analysis (see Table 6) was performed to investigate which cognitive measures are likely to best predict group membership (0 = HIV, 1 = Control). Since there are high correlations between the cognitive measures, the backward elimination procedure was chosen based on the Wald statistic. Considering Table 6, it can be observed that the cognitive variables that best predict group membership are SDMT (β = 0.055, χ2 = 4.338, p = .037) and semantic fluency (β = 0.061, χ2 = 6.360, p = .012). The positive β coefficients indicate that the higher the SDMT and the semantic fluency of a given individual, the more likely the corresponding individual has a normal performance. More specifically, if SDMT is increased by 1 unit, then we expect about 5.9% increase in the odds of being in the control category. Similarly, for a 1-unit increase in semantic fluency, we expect to see about 6.3% increase in the odds of being in the control class.
| – | β | SΕ β | Wald’s χ2 | df | p | eβ odds ratio |
|---|---|---|---|---|---|---|
| Constant | -6.685 | 1.831 | 13.333 | 1 | <.001* | NA |
| SDMT | 0.055 | 0.028 | 4.338 | 1 | .037* | 1.059 |
| Semantic fluency | 0.061 | 0.024 | 6.360 | 1 | .012* | 1.063 |
| Test | – | – | χ2 | df | p | – |
| Hosmer & Lemeshow | https://web.whatsapp.com/ | https://web.whatsapp.com/ | 5.498 | 8 | .703 | – |
4. DISCUSSION
The current study investigated the relationship between neurocognitive performance and complex language (syntax) abilities in a group of HIV-positive males on HAART, and whether performance across domains was associated with HIV infection-related factors and/or age and educational levels. This is the first research endeavor to our knowledge to have complex language abilities (syntax) amalgamated into the behavioral analysis of impaired neurocognitive performance in HIV seropositivity.
4.1. Group Comparisons for Neurocognitive and Linguistic Performance
The performance of the HIV-positive group in terms of neuropsychological and linguistic measurescompared to a control group matched to the target group for age, education and ethnic background. It is important to point out that even though the target group performed significantly worse than the control group on the Mini-Mental State Examination (MMSE), the performance was within the normal range for both the groups. At individual level, no HIV-positive participant was below the cut-off score on the MMSE. It is possible that the MMSE is not a suitable tool for diagnosing HAND [33], but in this case, we used a screening instrument to rule out dementia. There is new research suggesting that the Montreal Cognitive Assessment (MOCA) may be a more accurate screening tool for HAND in the clinical setting [34].
With regard to the overall findings, compared to the control group, the HIV-positive group showed significant difficulties in neuropsychological measures on the verbal modality, irrespective of whether they were storage or processing based. Specifically, HIV-positive participants showed deficits in verbal learning and episodic memory (measured by the RAVLT), in attention and speed of information processing and working memory (based on the SDMT), and lexical retrieval based on verbal fluency (both semantic category and phonemic (letter) based) performance. The results are in line with recent findings from larger-scale international cohorts screening for neurocognitive impairment in HIV [19, 33, 35-38]. Moreover, given that no HIV-positive participant reported being moderately-severe depressed, depression cannot be a factor associated with poorer verbal learning/memory or processing speed. This finding is consistent with the previous research revealing that depression and cognitive impairment are potentially independent in HIV [19] and in males with HIV [4].
On the other hand, the HIV-positive and control groups did not differ on executive function (EF) abilities (as measured by the TMTB and the SNST) including response inhibition and set-shifting. Certain aspects of EF abilities are shown to be comparatively unexplored in the previous research [4] but the opposite, that is, cognitive deterioration for executive functions, is also reported for HIV-positive populations [35, 37, 38]. Similarly, there were no differences between the HIV-positive and the control group on tasks that involved visuospatial modality (TMTA & LOT). This finding is not in line with recent results of a trail making test [12] or findings from more specialized measures of visuospatial abilities (e.g., hierarchical pattern perception) in HIV [11].
Overall, findings in the literature appear to be confounded by what researchers consider neuropsychological assessment measures are measuring exactly. For example, verbal fluency measures are used in some studies as a measure of language abilities [19, 33, 39], and in other studies as executive functions, as shown in studies cited by Kamminga et al. [40], and for other research as a measure of both executive functions and language abilities [38, 41]. The wide variations in the number and types of tasks that make up the neuropsychological battery, the content of the questionnaire-based measures and the method of administration (e.g., paper and pencil vs. computerized) present significant challenges to the interpretation of available data on NCI. It is crucial that common, informative screening tasks be proposed to simplify the choice of measures, minimize unnecessary testing, make procedures cost-effective [8], and enable researchers and clinicians to share a mutual point of reference regarding HAND diagnostics across languages and countries.
Moreover, this study is the first to incorporate a brief measure of complex language as part of the neuropsychological screening battery for NCI in a cohort of HIV-positive males. The SRT measure used probed implicit syntactic knowledge for subject-verb-object structures, object wh-questions, biclausal sentences involving coordination or subordination, object relative clauses, adverbial clauses, negation, and clitic left dislocation or clitic doubling (CLLD / CLD). Overall, the sentence repetition abilities of the HIV-cohort were observed to be significantly impaired compared to the control group. The HIV group made significantly more errors in function words (carry grammatical meaning) than the control group. On the other hand, function words (carry real meaning) appeared less impaired with regard to the number of omissions and word order errors.
Complex language deficits for the HIV-positive group, based on quantitative and qualitative error analyses, manifested only for operations involving negation and CLLD/CLD. The appropriate use of both negation and pronominal clitics (either in left dislocation or in doubling contexts) arguably entails more than mere syntactic computation and compositional semantics as compared to the other structures. These structures additionally link up to language-external pragmatic properties of context for identification and interpretation [42]. This kind of deficit could thus be linked to a problem in the syntax-discourse interface. It has been suggested that due to additional processing resources, the syntax-discourse interface is vulnerable in different pathologies such as in adults with stroke-induced aphasia [43] and Parkinson’s Disease [44] but also in children with specific language impairment or developmental language disorders [45]. This study provides new insights into the likelihood that the syntax-discourse interface may also be susceptible in HIV as a result of the pathological changes bought about by the virus to the frontostriatal and subcortical areas necessary for recruiting resources to foster complex language processing.
4.2. The Effects of Age and Education on Neurocognitive and Language Performance in HIV
Chronological age was shown to significantly correlate with performance on only one of the neurocognitive measures that is the RAVLT delay recall subtest and had no effect on syntactic sentence repetition abilities. This finding shows that the older individuals in the HIV-positive cohort had difficulties remembering and retrieving words (nouns) that they heard after a 20-minute delay, revealing deficits in episodic memory. Older age has been shown to be a potential risk factor for HIV-related NCI across several studies [4, 19, 33, 36, 46, 47], and a significant risk factor for memory impairments in HIV [48].
Years of education influenced the HIV-positive cohort’s performance on neurocognitive measures such as the MMSE, RAVLT delay recall, the TMTA and TMTB and for measures of verbal fluency (semantic and phonological). This means that individuals who are less educated performed poorly on these tasks. This finding is in line with the previous research showing that HIV-related NCI is associated with lower level of education [4, 19, 33, 36, 39, 46]. Similarly, years of education influenced performance in the sentence repetition task (both overall accuracy and repetition of target syntactic structure). Language abilities have been strongly correlated with levels of education across the lifespan, and that the language of adults with low education is characterized by simpler grammatical structures [49].
4.3. The Effects of the HIV Infection on Neurocognitive and Language Performance
Duration of HIV infection appeared to significantly contribute to verbal learning (encoding and consolidation), delay recall (i.e., retrieval), attention, visual scanning and speed of eye-hand coordination, set-shifting, processing speed, visuospatial function, and effective initiation skills (phonological fluency output). Individuals with a longer duration of infection performed worse on these measures. This is in line with the research that supports similar findings on disease duration as a risk factor for NCI (33,35,46]. Regarding the immunological variables, only lower nadir CD4+ T-cell counts were significantly associated with age. No other cognitive measure or sentence repetition abilities were influenced by HIV-related clinical variables. The finding that HIV disease markers (e.g., nadir CD4) were not significantly associated with neurocognitive performance is in agreement with recent research [4, 19, 33, 39, 47, 50]. It is important to point out that in the above-mentioned studies and in the current study, HIV-positive individuals were receiving antiretroviral treatment as standard care and showed viral suppression. Also, for our HIV cohort, there were minimal comorbid conditions (e.g., no drug abuse and non-positive hepatitis C) that may contribute to the development of NCI in HIV.
4.4. Limitations
There are several limitations to this study that deserve consideration. First, our HIV-positive cohort was exclusively all males (but this could also be a strength of the study). There is evidence that male gender is a risk factor for the development of HAND revealing that women may be more resilient or be equipped with an internal protective mechanism [33]. Second, there was large variability in the chronological age, level of education and duration of disease that could have influenced the NCI results for the HIV-positive cohort. Third, our results cannot be confirmed by neuroimaging data as we were unable to undertake neuroimaging methods to exclude diseases that can mimic HAND. Fourth, a stronger design would have sex-matched the clinical groups and control groups. Finally, we did not administer a quality of life (QoL) measure and did not have direct information on the presence/absence of early childhood language disorders and/or learning disabilities from case history information that could be premorbid prognostic indicators for change in language and cognitive status.
4.5. Summary
Mild neurocognitive impairments surfaced in HIV-positive individuals in cognitive domains such as verbal learning and memory, in attention and speed of information processing and working memory, and for verbal fluency performance. Also, based on a syntactic sentence repetition task, HIV-positive individuals showed difficulties in processing (repeating) certain complex syntactic operations. Yet, this HIV-positive group did not report problems with daily activities or complain of cognitive problems (ANI). This was consistent with the examiners’ observations based on case history information and questionnaire responses, that most individuals were independent with negligible functional limitations and no real complaints. In addition, the majority of participants (87.5%) reported being gainfully employed. This finding raises the possibility that more subtle forms of neurocognitive disorders associated with HIV persist even when individuals are on medication and that ANI does not represent disease-free baseline performance. It is crucial that the designation of “asymptomatic” be further investigated as it may be a more accurate reflection of researchers’ inability to document impaired function, potentially related to lack of appropriate, validated measures [21-23], for measuring neurocognitive change and outcomes for daily activity (floor effect), or that self-reporting of symptoms by patients may be unreliable [8]. Taken as a whole, the clinical significance of mild neurocognitive deficits and ANI still needs to be elucidated. The strengths of the present study include well-matched baseline data (HIV-positive group vs. control group), strict exclusion criteria, no comorbidities, and the use of a standardized and sensitive to HIV flexible neuropsychological battery. However, possible interpretive complications due to the sex differences within the control group as compared to the HIV-positive group are explicitly acknowledged.
CONCLUSION
The aim of this study was to address the growing need to understand the neuropsychological effects of HIV on neurocognitive abilities and complex language functioning. This is the first study to incorporate a brief expressive measure of complex sentence-level syntactic language abilities and to provide new insights into the syntax-discourse interface in HIV [42]. In our cohort, mild neurocognitive and linguistic impairments were examined in asymptomatic HIV-positive individuals who did not report problems with daily activities or complain of cognitive or language problems. Further research is urgently required regarding the relative contributions of each cognitive domain to language functions in HIV seropositive individuals. It is to hope that cognitive-linguistic research can further assist intervention practices [51-53].
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
The research and all procedures were approved by the Ethics Committee of the University Hospital of Patras, in Patras, and Laiko University Hospital, in Athens, where patients were recruited.
HUMAN AND ANIMAL RIGHTS
No animals were used in this research. All human research procedures followed were in accordance with the ethical standards of the committee responsible for human experimentation (institutional and national), and with the Helsinki Declaration of 1975, as revised in 2013.
CONSENT FOR PUBLICATION
Written informed consent was obtained from both the groups of participants involved in the study.
AVAILABILITY OF DATA AND MATERIALS
The data sets analyzed during the current study are available from the corresponding author upon request.
FUNDING
None.
CONFLICT OF INTEREST
The authors declare no conflict of interest, financial or otherwise.
ACKNOWLEDGEMENTS
We express our gratitude to all participants that took part in the study. The first author would like to thank the audience of the 10th European Congress of Speech and Language Therapy and the Speech Pathology Australia National Conference 2018, for very useful feedback on several aspects of the research. We express thanks to Nikoletta Christou and Dr. Ioannis Phinikettos for carrying out the statistical analyses. Finally, we acknowledge Maria Martzoukou and Nikoletta Christou for carrying out the auditory transcriptions.


